
A bridging ligand possess:
(This question has multiple correct answers)
A.Poly dentate nature
B.Two or more donor centres
C.The tendency to get itself attached to two metal ions
D.One donor centre
Answer
582.3k+ views
Hint: Bridging ligands can be basically explained as ligands that are connecting or joining or two or more atoms together. These ligands are usually used to connect metal ions. Now, these ligands may either be mono – atomic or polyatomic in nature.
Complete step by step answer:
Let us understand some facts and properties about bridging ligands:
In theory, almost all complex organic compounds can possibly be categorised as bridging ligands. So to avoid confusion, only small ligands like pseudo halides which are specifically designed to link two metals, fall into the category of bridging ligands.
Since bridging ligands are supposed to link two metal ions, it should at least have a bidentate nature. This means that it should have the capacity of at least forming bonds with two other species. This means that it has the capacity of being polydentate in nature. Also, to simply show the molecular representation of a bridging ligand:
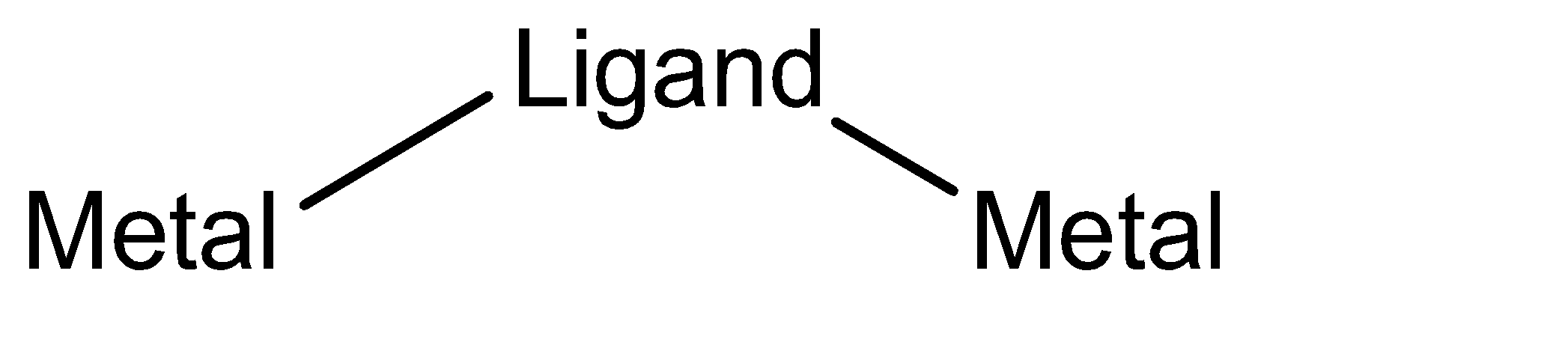
From the above data, we can conclude that a bridging ligand possesses a polydentate nature as well as has the tendency to get itself attached to two metal ions
Hence, Options A and C are the correct options.
Note:
Polyfunctional ligands can attach to metals in many ways and thus can bridge metals in diverse ways, including sharing of one atom or using several atoms. A classic example of a monodentate bridging ligand complex is the Creutz-Taube ion (1), \[{[{(N{H_3})_5}Ru(pz)Ru{(N{H_3})_5}]^{5 + }}\] where pz = pyrazine. This complex illustrates a typical mode of binding for a monodentate bridging ligand, pyrazine, coordinated here to two ruthenium metal centres.
Complete step by step answer:
Let us understand some facts and properties about bridging ligands:
In theory, almost all complex organic compounds can possibly be categorised as bridging ligands. So to avoid confusion, only small ligands like pseudo halides which are specifically designed to link two metals, fall into the category of bridging ligands.
Since bridging ligands are supposed to link two metal ions, it should at least have a bidentate nature. This means that it should have the capacity of at least forming bonds with two other species. This means that it has the capacity of being polydentate in nature. Also, to simply show the molecular representation of a bridging ligand:

From the above data, we can conclude that a bridging ligand possesses a polydentate nature as well as has the tendency to get itself attached to two metal ions
Hence, Options A and C are the correct options.
Note:
Polyfunctional ligands can attach to metals in many ways and thus can bridge metals in diverse ways, including sharing of one atom or using several atoms. A classic example of a monodentate bridging ligand complex is the Creutz-Taube ion (1), \[{[{(N{H_3})_5}Ru(pz)Ru{(N{H_3})_5}]^{5 + }}\] where pz = pyrazine. This complex illustrates a typical mode of binding for a monodentate bridging ligand, pyrazine, coordinated here to two ruthenium metal centres.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




