
A compound contains elements X and Y. Y atoms form CCP lattice and atoms of X occupy 1/3rd of tetrahedral voids. What is the molecular formula of the compound?
A.$XY$
B.$\mathop X\nolimits_2 \mathop Y\nolimits_3 $
C.$\mathop X\nolimits_3 \mathop Y\nolimits_2 $
D.\[\mathop {XY}\nolimits_2 \]
Answer
586.2k+ views
Hint: As we have studied in three dimensional arrangement i.e CCP or HCP, The number of octahedral voids present in the lattice is equal to the number of lattice points and the number of tetrahedral voids is twice the number of lattice points.
Complete step by step answer:
Position of Tetrahedral Voids in CCP or FCC Lattice: Let a unit cell be divided into eight small cubes in which each small cube has atoms at alternate corners. It means there are 4 atoms in each small cube, which form a regular tetrahedron when joined together. In this tetrahedron there is a tetrahedral void. In whole there are 8 tetrahedral voids in all 8 tetrahedrons. Hence, we conclude that the number of tetrahedral voids is double the number of lattice points.
So now,
No. of tetrahedral voids in CCP = \[2 \times {\text{ }}no.{\text{ }}of{\text{ }}atoms{\text{ }}of{\text{ }}Y\]
Atoms of Y in CCP unit cell is always = 4
So tetrahedral void = \[2 \times {\text{ }}4{\text{ }} = {\text{ }}8\]
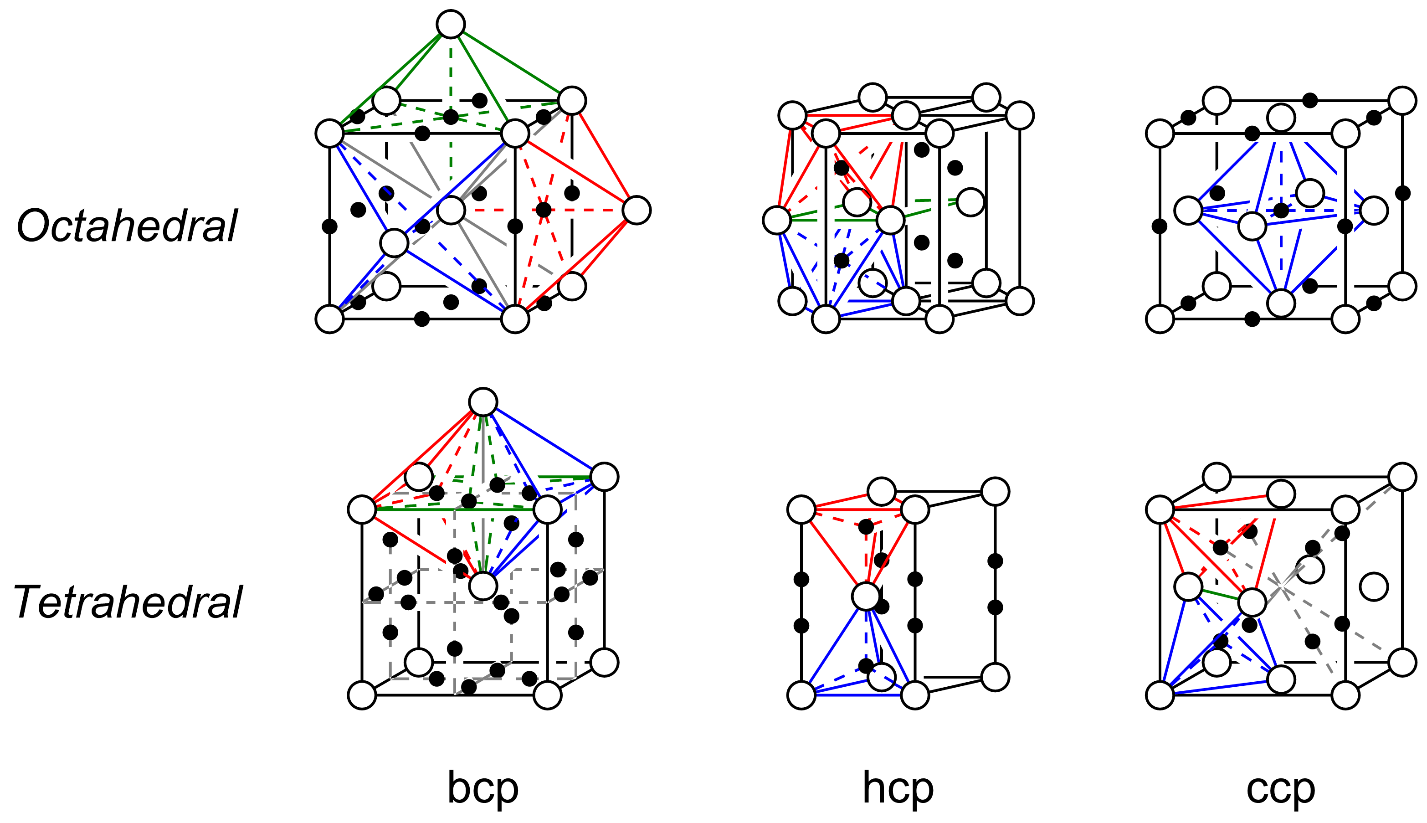
Number of tetrahedral voids occupied by element $x = \dfrac{1}{3} \times 8$
Now ratio of element X with that of $y = \dfrac{8}{3} + \dfrac{1}{4}$
The ratio comes to be \[2:3\]
Hence the formula will be $\mathop X\nolimits_2 \mathop Y\nolimits_3 $.
Hence option B is correct.
Note:
In ionic solids generally bigger ions i.e., anions form CCP and smaller ions i.e., cations occupy the voids. In a given compound some fraction of tetrahedral or octahedral voids are occupied by cations.
Complete step by step answer:
Position of Tetrahedral Voids in CCP or FCC Lattice: Let a unit cell be divided into eight small cubes in which each small cube has atoms at alternate corners. It means there are 4 atoms in each small cube, which form a regular tetrahedron when joined together. In this tetrahedron there is a tetrahedral void. In whole there are 8 tetrahedral voids in all 8 tetrahedrons. Hence, we conclude that the number of tetrahedral voids is double the number of lattice points.
So now,
No. of tetrahedral voids in CCP = \[2 \times {\text{ }}no.{\text{ }}of{\text{ }}atoms{\text{ }}of{\text{ }}Y\]
Atoms of Y in CCP unit cell is always = 4
So tetrahedral void = \[2 \times {\text{ }}4{\text{ }} = {\text{ }}8\]

Number of tetrahedral voids occupied by element $x = \dfrac{1}{3} \times 8$
Now ratio of element X with that of $y = \dfrac{8}{3} + \dfrac{1}{4}$
The ratio comes to be \[2:3\]
Hence the formula will be $\mathop X\nolimits_2 \mathop Y\nolimits_3 $.
Hence option B is correct.
Note:
In ionic solids generally bigger ions i.e., anions form CCP and smaller ions i.e., cations occupy the voids. In a given compound some fraction of tetrahedral or octahedral voids are occupied by cations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




