
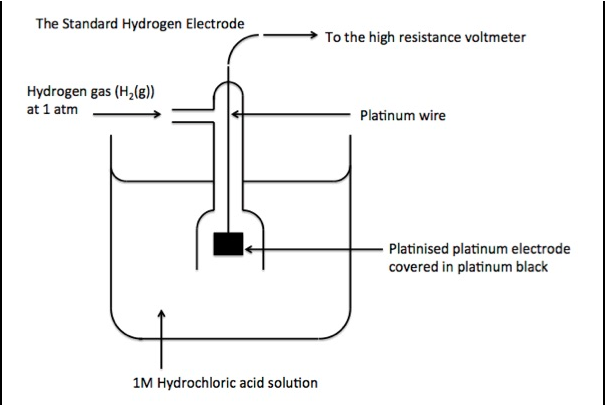
a) Draw Labelled diagram of Standard Hydrogen Electrode (SHE). Write its half-cell reaction of ${{\text{E}}^{0}}$ value.
b) Calculate $\Delta r{{G}^{0}}$ for the following reaction:
$F{{e}^{+2}}_{\left( aq \right)}+A{{g}^{+}}\to F{{e}^{+3}}_{(aq)}+A{{g}_{\left( s \right)}}$, (Given$E_{\left( cell \right)}^{0}=+0.03V,F=96500C$)
Answer
546.3k+ views
Hint: The Standard Hydrogen Electrode, is a redox electrode which is the basic of the thermodynamic scale of oxidation-reduction potential. It is basically used as a reference electrode on half-cell potential reactions. The value of the standard electrode potential is zero, which forms the basis to calculate cell potentials using different electrodes or different concentrations.
Complete answer:
The Standard Hydrogen Electrode also known as SHE. The potential of SHE is always 0 at 298K, this is the reason it is used as a reference electrode.
The redox half-cell reaction of the SHE is:
$2{{H}^{+}}_{\left( aq \right)}+2{{e}^{-}}\to {{H}_{2}}_{\left( g \right)}$${{E}^{0}}$
Hydrogen Standard electrode potential (${{E}^{0}}$) is declared as zero at any temperature.
Addition Information:
The absolute electrode potential of SHE is measured to be 4.44+-0.02V at room temperature. But for a basic comparison with all other electrode reactions, Hydrogen standard electrode potential (${{E}^{0}}$) is declared to be zero volts at any temperature.
The reasons for choice for platinum for the hydrogen electrode is:
-Inertness of platinum
-The capability of platinum to catalyze the reaction of proton reduction
-Use a surface material that absorbs hydrogen well at its interface. This increases the reaction kinetics
Note:
The value of standard electrode potential is always zero. During the reaction, hydrogen gas is passed through and into the solution and the reaction that takes place is: $2{{H}^{+}}_{\left( aq \right)}+2{{e}^{-}}\to {{H}_{2}}_{\left( g \right)}$
b) Calculate $\Delta r{{G}^{0}}$ for the following reaction:
$F{{e}^{+2}}_{\left( aq \right)}+A{{g}^{+}}\to F{{e}^{+3}}_{(aq)}+A{{g}_{\left( s \right)}}$, (Given$E_{(cell)}^{0}=+0.03V,F=96500C$)
Complete answer:
The Standard Hydrogen Electrode also known as SHE. The potential of SHE is always 0 at 298K, this is the reason it is used as a reference electrode.
The redox half-cell reaction of the SHE is:
$2{{H}^{+}}_{\left( aq \right)}+2{{e}^{-}}\to {{H}_{2}}_{\left( g \right)}$${{E}^{0}}$
Hydrogen Standard electrode potential (${{E}^{0}}$) is declared as zero at any temperature.

Addition Information:
The absolute electrode potential of SHE is measured to be 4.44+-0.02V at room temperature. But for a basic comparison with all other electrode reactions, Hydrogen standard electrode potential (${{E}^{0}}$) is declared to be zero volts at any temperature.
The reasons for choice for platinum for the hydrogen electrode is:
-Inertness of platinum
-The capability of platinum to catalyze the reaction of proton reduction
-Use a surface material that absorbs hydrogen well at its interface. This increases the reaction kinetics
Note:
The value of standard electrode potential is always zero. During the reaction, hydrogen gas is passed through and into the solution and the reaction that takes place is: $2{{H}^{+}}_{\left( aq \right)}+2{{e}^{-}}\to {{H}_{2}}_{\left( g \right)}$
b) Calculate $\Delta r{{G}^{0}}$ for the following reaction:
$F{{e}^{+2}}_{\left( aq \right)}+A{{g}^{+}}\to F{{e}^{+3}}_{(aq)}+A{{g}_{\left( s \right)}}$, (Given$E_{(cell)}^{0}=+0.03V,F=96500C$)
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




