
(a) Draw the structure of major monohalo products in each of the reaction.(refer diagram).
(b) Which halogen compound in each of the following pairs will react faster in \[{S_N}2\] reaction:
(i) \[C{H_3}Br\] or \[C{H_3}I\]
(ii) \[{(C{H_3})_3}C - Cl\] or \[C{H_3} - Cl\]
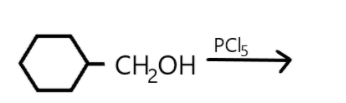
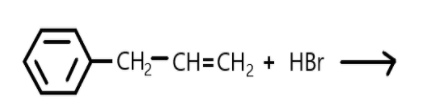
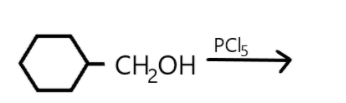
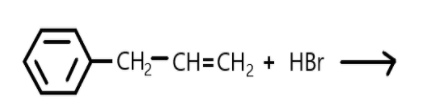
Answer
569.7k+ views
Hint: We will draw the diagram of a monhalo compound, based on their placement in phenyl. For the second part we will use the mechanism of \[{S_N}2\] reaction and electron affinity to find the faster reacting compound.
Complete step by step answer:
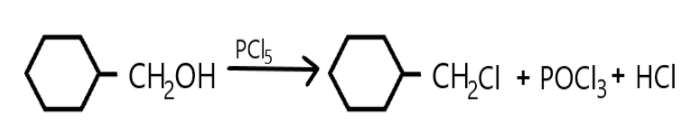
a. A compound is classified as a monohalo compound when the hydrogen atom on carbon atom is replaced by the halogen. Depending upon how the carbon hybridized the compound will form a monohalo structure. Similarly in the given reaction we can see that hydrogen atom is replaced by chlorine from $PC{l_5}$ and resulted in hydrochloric acid production as well as the oxygen atom is replaced which resulted in $POC{l_3}$.
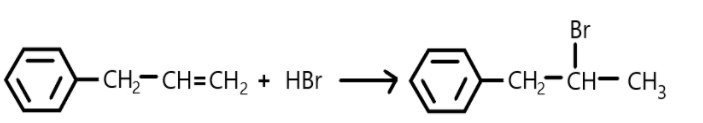
In this reaction the double bond is broken, and halogen is added on carbon.
b. \[{S_N}2\] reaction is a nucleophilic substitution reaction in which the reactions are bimolecular with simultaneous bond formation and breakage. It doesn’t require an intermediate to facilitate the reaction. The final product produced after \[{S_N}2\] reactions are stereochemistry inversion at their centre and have steric effect.
i. Between \[C{H_3}Br\] or \[C{H_3}I\], \[C{H_3}I\] will react faster to the \[{S_N}2\] reaction because Iodine present in the compound will leave easily due to large atomic size compared to atomic size of bromine.
ii. Between \[{(C{H_3})_3}C - Cl\] or \[C{H_3} - Cl\], \[C{H_3} - Cl\] will react faster to the \[{S_N}2\] reaction because \[C{H_3} - Cl\] is a primary halide and \[{(C{H_3})_3}C - Cl\] is tertiary halide and we know that primary halide reacts faster to \[{S_N}2\] reaction.
Note:
Student will make mistakes while making the monohalo diagrams of the compounds and they will also make mistakes in selecting the properties for elements which react faster in the \[{S_N}2\] reaction.
Complete step by step answer:
a. A compound is classified as a monohalo compound when the hydrogen atom on carbon atom is replaced by the halogen. Depending upon how the carbon hybridized the compound will form a monohalo structure. Similarly in the given reaction we can see that hydrogen atom is replaced by chlorine from $PC{l_5}$ and resulted in hydrochloric acid production as well as the oxygen atom is replaced which resulted in $POC{l_3}$.

In this reaction the double bond is broken, and halogen is added on carbon.

b. \[{S_N}2\] reaction is a nucleophilic substitution reaction in which the reactions are bimolecular with simultaneous bond formation and breakage. It doesn’t require an intermediate to facilitate the reaction. The final product produced after \[{S_N}2\] reactions are stereochemistry inversion at their centre and have steric effect.
i. Between \[C{H_3}Br\] or \[C{H_3}I\], \[C{H_3}I\] will react faster to the \[{S_N}2\] reaction because Iodine present in the compound will leave easily due to large atomic size compared to atomic size of bromine.
ii. Between \[{(C{H_3})_3}C - Cl\] or \[C{H_3} - Cl\], \[C{H_3} - Cl\] will react faster to the \[{S_N}2\] reaction because \[C{H_3} - Cl\] is a primary halide and \[{(C{H_3})_3}C - Cl\] is tertiary halide and we know that primary halide reacts faster to \[{S_N}2\] reaction.
Note:
Student will make mistakes while making the monohalo diagrams of the compounds and they will also make mistakes in selecting the properties for elements which react faster in the \[{S_N}2\] reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




