
A hydrocarbon of molecular formula ${{C}_{5}}{{H}_{10}}$ on monochlorination gives one product and on chlorination gives three products (excluding the stereoisomers). Identify the hydrocarbon.
Answer
585.9k+ views
Hint: It is an alicyclic hydrocarbon and a highly flammable compound. This compound is made by the cracking of cyclohexane in the presence of high temperature and pressure.
Complete step by step answer:
- This question is done basically by a hit and trial method.
- From the first part of the question which is the molecular formula, ${{C}_{5}}{{H}_{10}}$ comes under the general formula of ${{C}_{n}}{{H}_{2n}}$.
- From here we can get to know that the compound is either an alkene or a cyclic compound.
- So, the possible molecules are pentene and cyclopentane.
- From this point, we can look at the second part of the question, which says that the monochlorination of this compound should give only one product.
- In case of pentene (whether it is 1-pentene or 2-pentene), there are two $\alpha $-hydrogens and so, on monochlorination, there will be two different products formed.

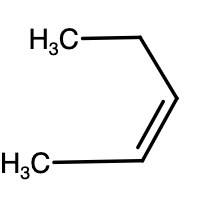
- Above given are the structures of pentene. The reaction is given as,
\[C{{H}_{2}}=CHC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}+HCl\to Cl-C{{H}_{2}}-C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}+C{{H}_{3}}-\underset{\underset{Cl}{\mathop{|}}\,}{\mathop{CH}}\,-C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
-As we can see above, monochlorination of 1-pentene results in two different products. Similar reaction will take place for 2-pentene as well.
- So, we can now check the monochlorination product of cyclopentane.
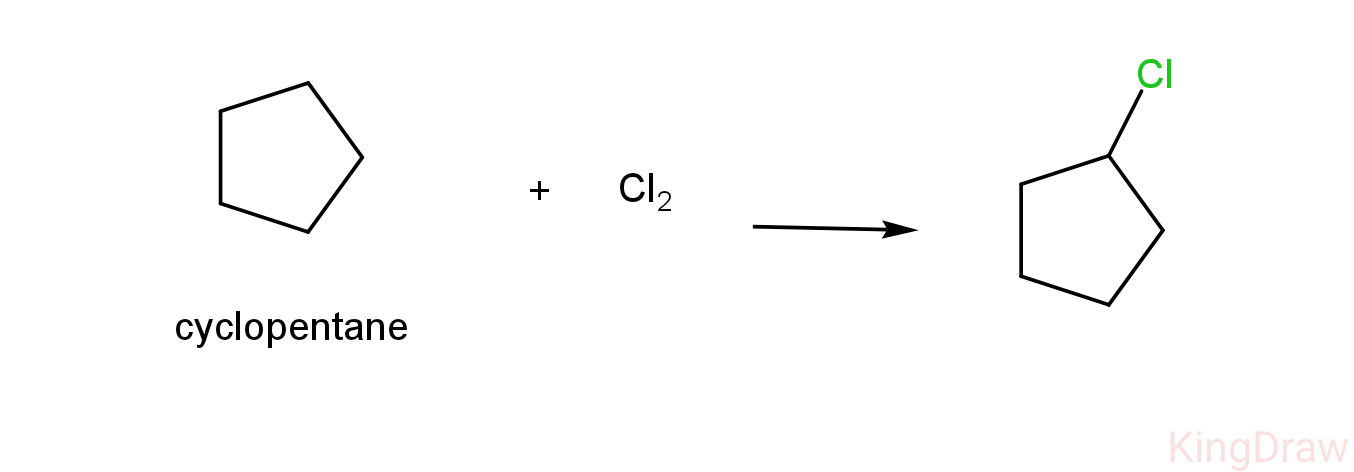
-As we can see above, cyclopentane reacts with chlorine to form only one product which is 1-chlorocyclopentane.
- Now, to confirm the compound, we can see the next part of the question which says that on dichlorination it should give three products
- Given below is the dichlorination of cyclopentane.
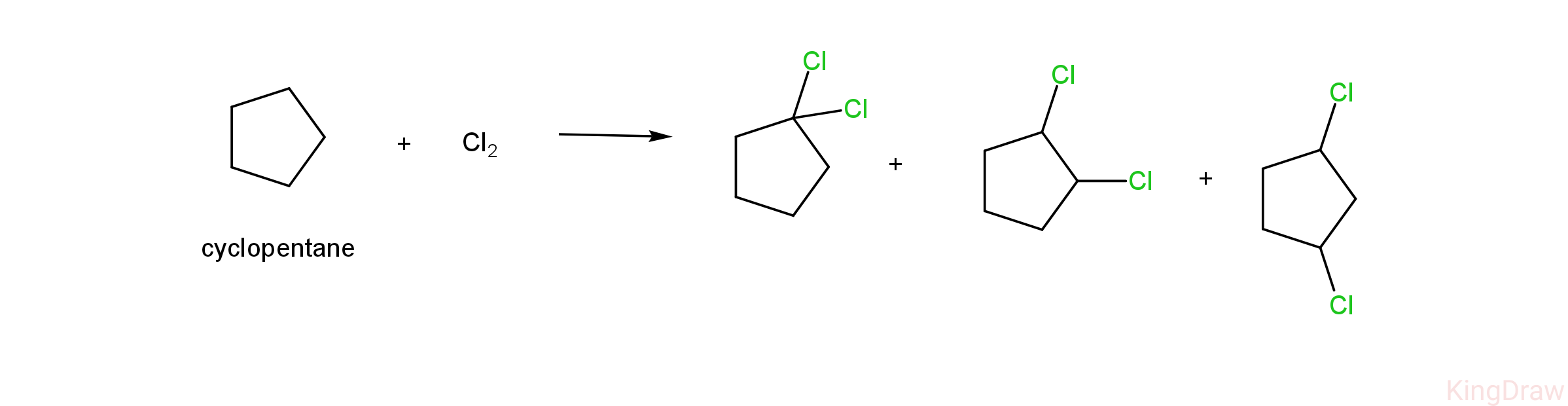
- As we can see, the dichlorination of cyclopentane results in the formation of three products, which are 1,1-dichlorocyclopentane, 1,2 -dichlorocyclopentane and 1,3-dichlorocyclopentane.
- Therefore , we can conclude that cyclopentane is the compound having the molecular formula of C5H10, on monochlorination giving one product (1-chloro cyclopentane) and on dichlorination gives three products (1,1-dichloro cyclopentane, 1,2 -dichlorocyclopentane and 1,3- dichlorocyclopentane)
- So, the answer is Cyclopentane.
Note: Monochlorination and dichlorination both the reaction proceeds under different sets of conditions. Monochlorination of cyclopentane occurs with $C{{l}_{2}}$ in the presence of light ($h\upsilon $) whereas dichlorination of cyclopentene occurs when excess of $C{{l}_{2}}$ is provided in the presence of light ($h\upsilon $).
Complete step by step answer:
- This question is done basically by a hit and trial method.
- From the first part of the question which is the molecular formula, ${{C}_{5}}{{H}_{10}}$ comes under the general formula of ${{C}_{n}}{{H}_{2n}}$.
- From here we can get to know that the compound is either an alkene or a cyclic compound.
- So, the possible molecules are pentene and cyclopentane.
- From this point, we can look at the second part of the question, which says that the monochlorination of this compound should give only one product.
- In case of pentene (whether it is 1-pentene or 2-pentene), there are two $\alpha $-hydrogens and so, on monochlorination, there will be two different products formed.


- Above given are the structures of pentene. The reaction is given as,
\[C{{H}_{2}}=CHC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}+HCl\to Cl-C{{H}_{2}}-C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}+C{{H}_{3}}-\underset{\underset{Cl}{\mathop{|}}\,}{\mathop{CH}}\,-C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
-As we can see above, monochlorination of 1-pentene results in two different products. Similar reaction will take place for 2-pentene as well.
- So, we can now check the monochlorination product of cyclopentane.


-As we can see above, cyclopentane reacts with chlorine to form only one product which is 1-chlorocyclopentane.
- Now, to confirm the compound, we can see the next part of the question which says that on dichlorination it should give three products
- Given below is the dichlorination of cyclopentane.

- As we can see, the dichlorination of cyclopentane results in the formation of three products, which are 1,1-dichlorocyclopentane, 1,2 -dichlorocyclopentane and 1,3-dichlorocyclopentane.
- Therefore , we can conclude that cyclopentane is the compound having the molecular formula of C5H10, on monochlorination giving one product (1-chloro cyclopentane) and on dichlorination gives three products (1,1-dichloro cyclopentane, 1,2 -dichlorocyclopentane and 1,3- dichlorocyclopentane)
- So, the answer is Cyclopentane.
Note: Monochlorination and dichlorination both the reaction proceeds under different sets of conditions. Monochlorination of cyclopentane occurs with $C{{l}_{2}}$ in the presence of light ($h\upsilon $) whereas dichlorination of cyclopentene occurs when excess of $C{{l}_{2}}$ is provided in the presence of light ($h\upsilon $).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




