
a) i) Explain the preparation of phenol from cumene.
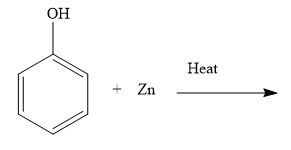
ii) Complete the reaction (please find above image).
b) Explain Williamson's ether synthesis.

Answer
569.1k+ views
Hint:a)
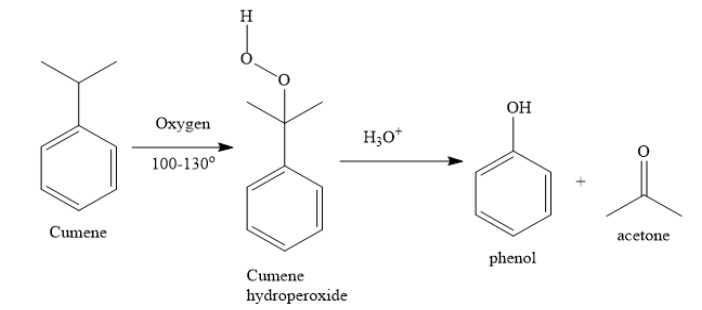
i) Cumene is isopropyl benzene. When you convert cumene to phenol, you need an oxidation reaction.
ii) You exchange an oxygen atom between phenol and zinc.
b) In Williamson ether synthesis, you react to two different substrates to prepare an ether.
Complete answer:
a) i) You can prepare phenol from cumene by air oxidation, followed by acidic hydrolysis. Cumene is isopropyl benzene. Acetone is the side product.
ii) When you heat phenol with zinc, you obtain benzene and zinc oxide. Write the chemical reaction as shown below:
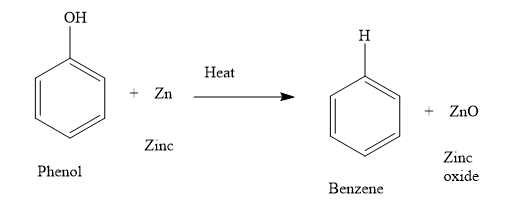
The oxygen atom of phenol is now with zinc. Thus, during the reaction, phenol is reduced and zinc is oxidized. Loss of oxygen is reduction and gain of oxygen is oxidation. The oxidation number of zinc increases from 0 to +2. Increase in the oxidation number is the oxidation and decrease in the oxidation number is reduction.
b) In the Williamson ether synthesis, you prepare an ether from organo halides and a deprotonated alcohol (alkoxide).

Thus, you react sodium ethoxide with ethyl chloride to obtain diethyl ether. Sodium chloride is the by-product.
Note:
a) i) Apart from the preparation of phenol from cumene, there are various other methods by which you can prepare phenol.
ii) In the redox reaction, one substance is reduced and acts as an oxidizing agent. The other substance is oxidized and acts as a reducing agent.
b) With the help of Williamson's ether synthesis, you can prepare simple ethers and mixed ethers. However the purification of mixed ethers is complicated.
i) Cumene is isopropyl benzene. When you convert cumene to phenol, you need an oxidation reaction.
ii) You exchange an oxygen atom between phenol and zinc.
b) In Williamson ether synthesis, you react to two different substrates to prepare an ether.
Complete answer:
a) i) You can prepare phenol from cumene by air oxidation, followed by acidic hydrolysis. Cumene is isopropyl benzene. Acetone is the side product.

ii) When you heat phenol with zinc, you obtain benzene and zinc oxide. Write the chemical reaction as shown below:

The oxygen atom of phenol is now with zinc. Thus, during the reaction, phenol is reduced and zinc is oxidized. Loss of oxygen is reduction and gain of oxygen is oxidation. The oxidation number of zinc increases from 0 to +2. Increase in the oxidation number is the oxidation and decrease in the oxidation number is reduction.
b) In the Williamson ether synthesis, you prepare an ether from organo halides and a deprotonated alcohol (alkoxide).

Thus, you react sodium ethoxide with ethyl chloride to obtain diethyl ether. Sodium chloride is the by-product.
Note:
a) i) Apart from the preparation of phenol from cumene, there are various other methods by which you can prepare phenol.
ii) In the redox reaction, one substance is reduced and acts as an oxidizing agent. The other substance is oxidized and acts as a reducing agent.
b) With the help of Williamson's ether synthesis, you can prepare simple ethers and mixed ethers. However the purification of mixed ethers is complicated.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




