
A. Major product of chlorination of ethyl benzene is
a. m-chloroethyl benzene
b. p - chloroethyl benzene
c. chlorobenzene
d. o-chloroethyl benzene
B. 1 - chloropropane on treatment with alcoholic potassium hydroxide produces
a. propane
b. propene
c. propyne
d. propyl alcohol
Answer
569.4k+ views
Hint:A. Different products will be obtained from different reagents. When chlorination is carried out in presence of iron or ferric chloride catalyst, the aromatic ring will be substituted. If chlorination is carried out in presence of heat or light, then alkyl side chains will be substituted.
B. In presence of alcoholic potassium hydroxide, dehydrohalogenation reaction occurs. In presence of aqueous potassium hydroxide substitution reaction occurs.
Complete answer:
A. When chlorination of ethylbenzene is carried out in presence of heat or light, 1-chloro-1-phenylethane is obtained as the major product. In this reaction, chlorine enters the alkyl side chain and aromatic benzene ring is not affected.
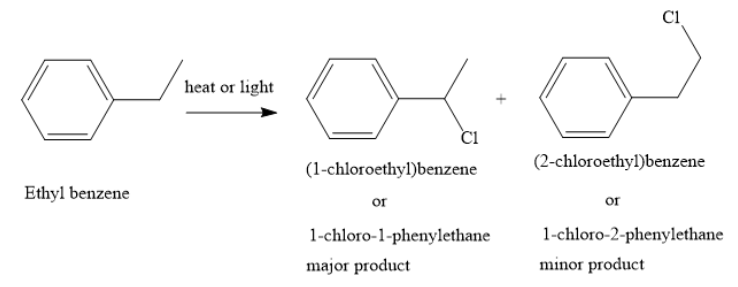
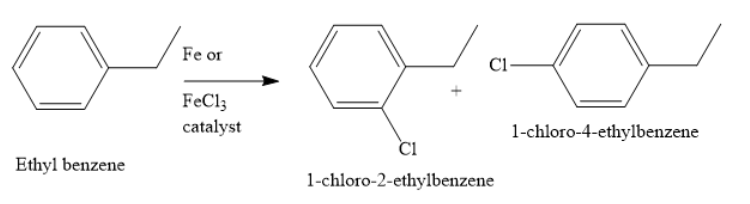
When chlorination of ethylbenzene is carried out in presence of iron or ferric chloride catalyst, the aromatic ring is substituted to form a mixture of o-chloroethyl benzene and p o-chloroethyl benzene. This is because the ethyl group is an ortho para directing group. Also, ethyl groups are activating in electrophilic aromatic substitution reactions.
Hence, the options b. and d. are correct options.
B. 1 - chloropropane on treatment with alcoholic potassium hydroxide produces propene
In presence of alcoholic potassium hydroxide, alkyl halides undergo dehydrohalogenation to produce an alkene. In this reaction, a molecule of hydrogen chloride is eliminated to form carbon-carbon double bonds.
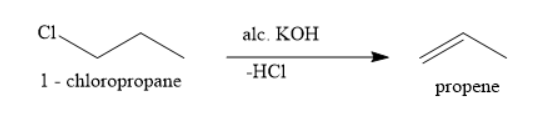
Hence, the correct option is the option b.
Note:
A. Aromatic nucleus prefers substitution over addition. Due to addition reaction of the aromatic ring, the aromatic stabilization will be lost and hence is not favoured.
B. In presence of aqueous potassium hydroxide substitution reaction occurs. 1-chloropropane, in reaction with aqueous potassium hydroxide will give 1-propanol.
B. In presence of alcoholic potassium hydroxide, dehydrohalogenation reaction occurs. In presence of aqueous potassium hydroxide substitution reaction occurs.
Complete answer:
A. When chlorination of ethylbenzene is carried out in presence of heat or light, 1-chloro-1-phenylethane is obtained as the major product. In this reaction, chlorine enters the alkyl side chain and aromatic benzene ring is not affected.

When chlorination of ethylbenzene is carried out in presence of iron or ferric chloride catalyst, the aromatic ring is substituted to form a mixture of o-chloroethyl benzene and p o-chloroethyl benzene. This is because the ethyl group is an ortho para directing group. Also, ethyl groups are activating in electrophilic aromatic substitution reactions.
Hence, the options b. and d. are correct options.

B. 1 - chloropropane on treatment with alcoholic potassium hydroxide produces propene
In presence of alcoholic potassium hydroxide, alkyl halides undergo dehydrohalogenation to produce an alkene. In this reaction, a molecule of hydrogen chloride is eliminated to form carbon-carbon double bonds.

Hence, the correct option is the option b.
Note:
A. Aromatic nucleus prefers substitution over addition. Due to addition reaction of the aromatic ring, the aromatic stabilization will be lost and hence is not favoured.
B. In presence of aqueous potassium hydroxide substitution reaction occurs. 1-chloropropane, in reaction with aqueous potassium hydroxide will give 1-propanol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




