
A phase diagram for solvents and solutions is shown in the figure. What represents the normal boiling point of the solution?

Answer
507k+ views
Hint: We are discussing the normal boiling point of the solution. We should know first what the normal boiling point is. Normal boiling point of a solution is the temperature at which the vapour pressure of the solution becomes equal to the atmospheric pressure. We are given the phase diagram for solvents and solutions. We will study the diagram to solve this question.
Complete answer:
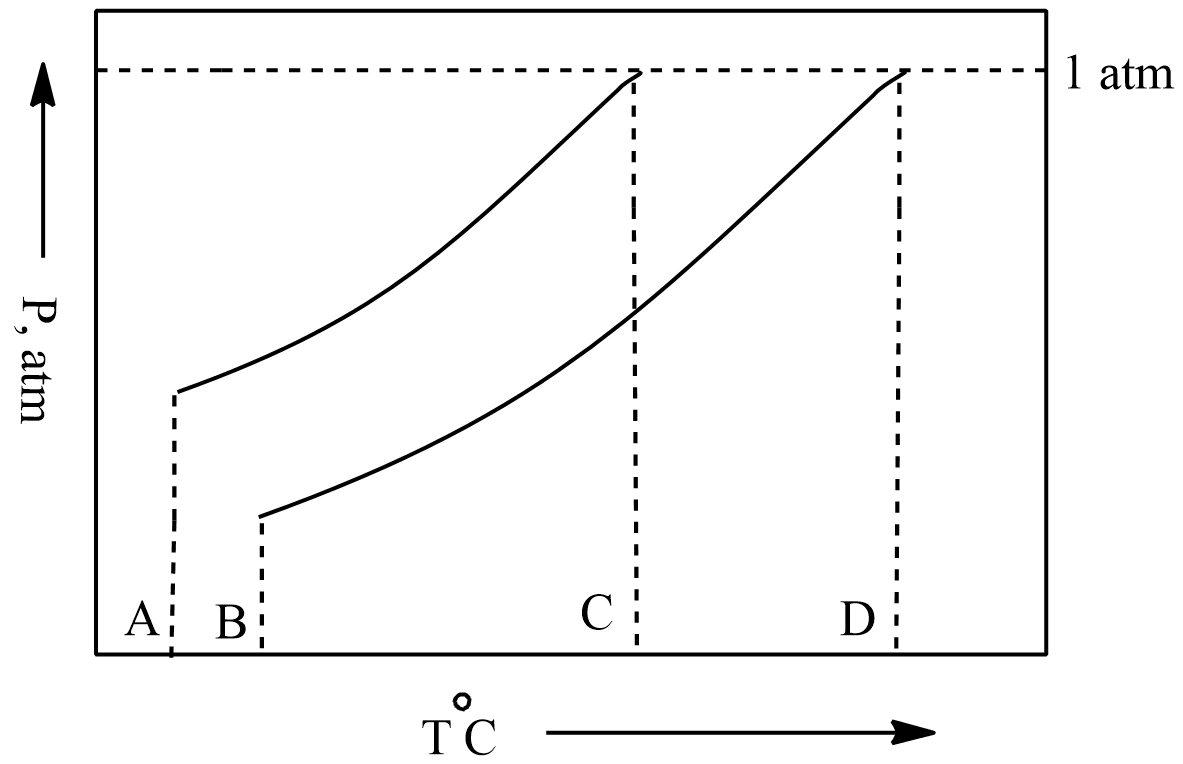
Phase diagrams are pressure vs temperature which is used to predict the state of the solvent and the solution.
Point A in the phase diagram is the initial state of solvent showing the temperature and pressure at that time.
Now when we see point B is the initial state of the solution, we can see that when we add any solute to pure solvent its pressure is decreased which can be seen in the phase diagram as pressure at point A was higher because it is of pure solvent.
Now we move on to point C. It is the state where the vapour pressure of the solvent becomes equal to the atmospheric pressure so point C represents the Boiling point of the solvent.
Now we are at the last point D. It is the state where the vapour pressure of the solution becomes equal to atmospheric pressure, so point D represents the normal boiling point of the solution.
So, Point D is the correct answer.
Note:
The difference between the Point D and Point C i.e., boiling point of the solution and boiling point of the solvent is known as the elevation in boiling point. It can be observed from the phase diagram that the boiling point of solution is higher than the boiling point of pure solvent.
Complete answer:
Phase diagrams are pressure vs temperature which is used to predict the state of the solvent and the solution.
Point A in the phase diagram is the initial state of solvent showing the temperature and pressure at that time.
Now when we see point B is the initial state of the solution, we can see that when we add any solute to pure solvent its pressure is decreased which can be seen in the phase diagram as pressure at point A was higher because it is of pure solvent.
Now we move on to point C. It is the state where the vapour pressure of the solvent becomes equal to the atmospheric pressure so point C represents the Boiling point of the solvent.
Now we are at the last point D. It is the state where the vapour pressure of the solution becomes equal to atmospheric pressure, so point D represents the normal boiling point of the solution.
So, Point D is the correct answer.
Note:
The difference between the Point D and Point C i.e., boiling point of the solution and boiling point of the solvent is known as the elevation in boiling point. It can be observed from the phase diagram that the boiling point of solution is higher than the boiling point of pure solvent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




