
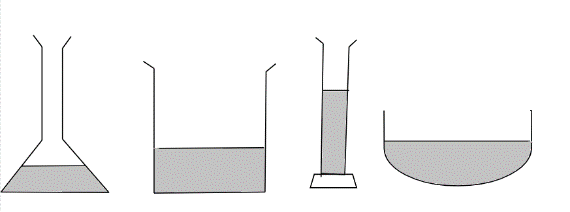
A student poured an equal amount of water into 4 containers as shown. What does this experiment show?
A. Water has definite volume
B. Water has no definite shape
C. Water has definite mass
D. None of the above

Answer
500.4k+ views
Hint: Water is a polar compound which is composed of the chemical elements hydrogen and oxygen in the ratio $2:1$. Water can exist in all the three states of matter i.e. solid, liquid or gas with the same chemical formula ${H_2}O$. Moreover, triple point is a temperature in thermodynamics at which water can exist in all the three states of matter.
Complete answer:
When the student poured equal amounts of water into four different containers, this experiment indicates that the height of the poured water in different containers is different because of the one of many physical properties of the water i.e. water has no definite shape, instead water takes the shape of the container in which it is kept. This property of water is due to the arrangement of particles in its liquid state where particles are in close contact yet having the freedom to move around. Also, particles of water in liquid state are held weakly by intermolecular forces, although this intermolecular force is strong enough to hold the particles together but not at fixed position.
This experiment hence indicates that water in its liquid state with chemical formula ${H_2}O$ does not have definite shape and it takes the shape of the container in which it is kept but the volume of water is definite.
So the correct answer is option B.
Note:
Water is also known as the universal solvent because of its ability to dissolve many substances. Water is known to have high heat capacity as well as high heat of vaporization. Moreover, the density of water in liquid state is less than its density in solid state. Water has a tetrahedral geometry.
Complete answer:
When the student poured equal amounts of water into four different containers, this experiment indicates that the height of the poured water in different containers is different because of the one of many physical properties of the water i.e. water has no definite shape, instead water takes the shape of the container in which it is kept. This property of water is due to the arrangement of particles in its liquid state where particles are in close contact yet having the freedom to move around. Also, particles of water in liquid state are held weakly by intermolecular forces, although this intermolecular force is strong enough to hold the particles together but not at fixed position.
This experiment hence indicates that water in its liquid state with chemical formula ${H_2}O$ does not have definite shape and it takes the shape of the container in which it is kept but the volume of water is definite.
So the correct answer is option B.
Note:
Water is also known as the universal solvent because of its ability to dissolve many substances. Water is known to have high heat capacity as well as high heat of vaporization. Moreover, the density of water in liquid state is less than its density in solid state. Water has a tetrahedral geometry.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Trending doubts
What is BLO What is the full form of BLO class 8 social science CBSE

Citizens of India can vote at the age of A 18 years class 8 social science CBSE

Full form of STD, ISD and PCO

Advantages and disadvantages of science

Right to vote is a AFundamental Right BFundamental class 8 social science CBSE

What are the 12 elements of nature class 8 chemistry CBSE




