
When acetamide is treated with $NaOBr$, the product formed is :
A) $C{H_3}CN$
B) $C{H_3}C{H_2}N{H_2}$
C) $C{H_3}N{H_2}$
D) None of these
Answer
582.3k+ views
Hint: This reaction of acetamide with $NaOBr$ is an example of Hofmann degradation of primary amides to amines with resultant compounds having one carbon atom less. It is an important method of preparation of amines and this reaction is also used to descend the series as the product amine contains one less carbon atom.
Complete step by step answer: As the name of the reaction suggests, Hoffmann bromamide degradation was given by August Von Hoffmann. It is also called the Hofmann rearrangement reaction.
So, understand complete meaning of Hoffmann bromamide synthesis
Hoffmann is the chemist name who proposed this mechanism.
Bromamide is a Bromine molecule and amide is used in the reaction.
Degradation – The one carbon is lesser in the product compared to reactant, so that degradation of product takes place.
$NaOBr$ is a compound and its chemical name is Sodium hypobromite.
Acetamide reacts with $NaOBr$ and gives methylamine. This reaction is also called as Hoffmann Bromamide synthesis
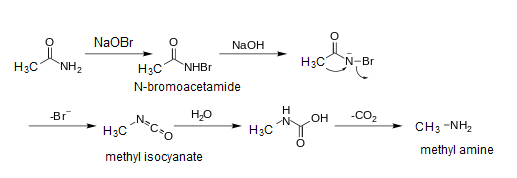
Step -$1$ In the above reaction the Acetamide first reacts with Sodium hypobromite and forms N-bromo-acetamide by the abstracting proton from the compound and forms a conjugate base. This conjugate base is called a bromamide.
Step -$2$ In this step sodium hydroxide reacts with bromamide and another hydrogen is removed and $C{H_3} - CO - NBr$ is left behind.
Step -$3$ In this step the Bromine is eliminated as Bromide ion and in this step the alkyl group is directly attached to the Nitrogen atom and forms directly methyl isocyanate.
Step -$4$ In this step Isocyanate reacts with water and removal of carbon dioxide takes place and forms methyl amine.
So, the first option name is methyl cyanide; it is not the answer.
Coming to the second option, its name is Ethyl amine.
Third option is Methylamine.
Hence the answer is (c) Methylamine.
Note:Sometimes this reaction actually uses Bromine and sodium hydroxide, but Sodium hypobromite is also equivalent with Bromine and Sodium hydroxide. This reaction proceeds with the formation of isocyanate intermediate.
Complete step by step answer: As the name of the reaction suggests, Hoffmann bromamide degradation was given by August Von Hoffmann. It is also called the Hofmann rearrangement reaction.
So, understand complete meaning of Hoffmann bromamide synthesis
Hoffmann is the chemist name who proposed this mechanism.
Bromamide is a Bromine molecule and amide is used in the reaction.
Degradation – The one carbon is lesser in the product compared to reactant, so that degradation of product takes place.
$NaOBr$ is a compound and its chemical name is Sodium hypobromite.
Acetamide reacts with $NaOBr$ and gives methylamine. This reaction is also called as Hoffmann Bromamide synthesis

Step -$1$ In the above reaction the Acetamide first reacts with Sodium hypobromite and forms N-bromo-acetamide by the abstracting proton from the compound and forms a conjugate base. This conjugate base is called a bromamide.
Step -$2$ In this step sodium hydroxide reacts with bromamide and another hydrogen is removed and $C{H_3} - CO - NBr$ is left behind.
Step -$3$ In this step the Bromine is eliminated as Bromide ion and in this step the alkyl group is directly attached to the Nitrogen atom and forms directly methyl isocyanate.
Step -$4$ In this step Isocyanate reacts with water and removal of carbon dioxide takes place and forms methyl amine.
So, the first option name is methyl cyanide; it is not the answer.
Coming to the second option, its name is Ethyl amine.
Third option is Methylamine.
Hence the answer is (c) Methylamine.
Note:Sometimes this reaction actually uses Bromine and sodium hydroxide, but Sodium hypobromite is also equivalent with Bromine and Sodium hydroxide. This reaction proceeds with the formation of isocyanate intermediate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




