
Acetic acid exists in a dimer state in benzene, due to _ _ _ _ _ _ _ _ _?
(A) Condensation reaction
(B) Hydrogen bonding
(C) Presence of carbonyl group
(D) Presence of α-hydrogen
Answer
584.7k+ views
Hint: Acetic acid is a polar molecule while benzene is a non-polar solvent. So, acetic acid cannot dissolve in benzene. Also remember there is a large electronegativity difference between hydrogen and oxygen.
Complete step by step answer:
-First of all let us see what acetic acid is and its structure.
Acetic acid is also known as ethanoic acid and has a molecular formula of $C{H_3}COOH$. It is a weak acid because it dissociates only partially in solution. It is a polar molecule. Its structure is:
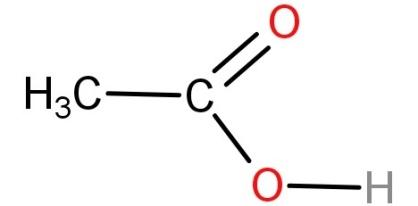
-Now let's talk about benzene.
It is an organic compound with molecular formula of ${C_6}{H_6}$. It is a six carbon planar ring and is a non-polar solvent. Its structure is:
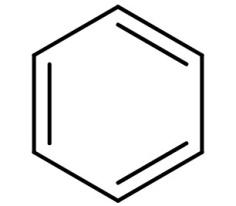
-We all know that polar molar dissolves in polar solvent and non-polar molecules dissolve in non-polar solvent. But a polar molecule cannot be dissolved in a nonpolar solvent.
Acetic acid being a polar molecule cannot be dissolved in the non-polar solvent benzene. So, the acetic acid molecules undergo intramolecular hydrogen bonding due to the electronegativity difference between hydrogen and oxygen. This leads to formation of acetic acid dimer molecules. Acetic acid is more stable in dimer state as compared to its single molecule or monomer form.
The dimer looks like:
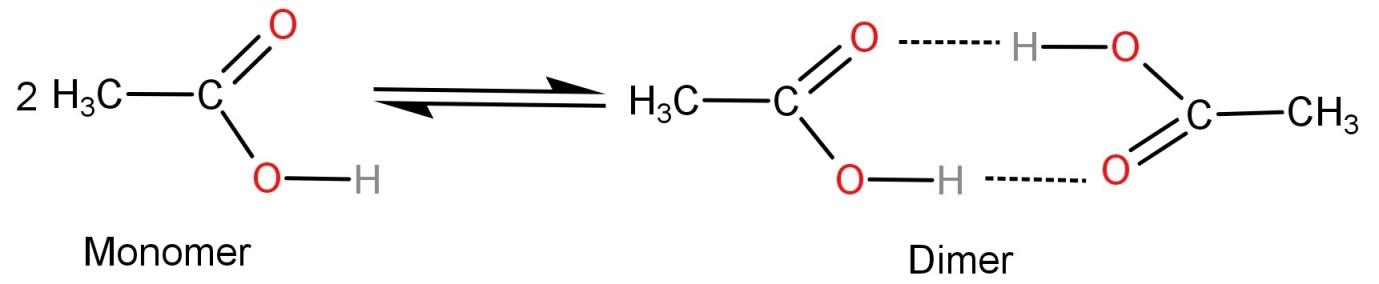
Hence the correct option is: (B) Hydrogen bonding.
Note: In undiluted form acetic acid is known as glacial acetic acid. When acetic acid is present as 4% by volume, it forms vinegar and hence we can say that acetic acid is the main component of vinegar. Acetic acid is also used as a monomer of vinyl acetate, for ester production, as a polar protic solvent and also for medicinal uses as antiseptic and cervical cancer screening.
Complete step by step answer:
-First of all let us see what acetic acid is and its structure.
Acetic acid is also known as ethanoic acid and has a molecular formula of $C{H_3}COOH$. It is a weak acid because it dissociates only partially in solution. It is a polar molecule. Its structure is:

-Now let's talk about benzene.
It is an organic compound with molecular formula of ${C_6}{H_6}$. It is a six carbon planar ring and is a non-polar solvent. Its structure is:

-We all know that polar molar dissolves in polar solvent and non-polar molecules dissolve in non-polar solvent. But a polar molecule cannot be dissolved in a nonpolar solvent.
Acetic acid being a polar molecule cannot be dissolved in the non-polar solvent benzene. So, the acetic acid molecules undergo intramolecular hydrogen bonding due to the electronegativity difference between hydrogen and oxygen. This leads to formation of acetic acid dimer molecules. Acetic acid is more stable in dimer state as compared to its single molecule or monomer form.
The dimer looks like:

Hence the correct option is: (B) Hydrogen bonding.
Note: In undiluted form acetic acid is known as glacial acetic acid. When acetic acid is present as 4% by volume, it forms vinegar and hence we can say that acetic acid is the main component of vinegar. Acetic acid is also used as a monomer of vinyl acetate, for ester production, as a polar protic solvent and also for medicinal uses as antiseptic and cervical cancer screening.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




