
Acetone reacts with iodine $({I_2})$ to from iodoform, in the presence of :
(A) \[KOH\]
(B) \[NaOH\]
(C) \[CuC{O_3}\]
(D) \[MgC{O_3}\]
Answer
578.4k+ views
Hint: Iodoform is chemically tri-iodo-methane its chemical formula is $CH{I_3}.$
Iodoform is prepared by ethyl alcohol and acetone in laboratories.
Complete step by step answer:
(1) Acetone is when treated with iodine and potassium hydroxide, producing iodoform.
When reaction takes place in following steps.
(2) In the first step iodine reacts with acetone in presence of $KOH$ and form tri-iodo-acetone.
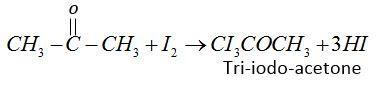
(3) Second step tri-iodo-ethane reacts with $KOH$and forms iodoform.
$C{I_3}COC{H_3} + KOH \to CH{I_3} + C{H_3}COOK$
Iodoform potassium acetate
Therefore, form the above explanation the correct option is (A) $KOH.$
About $3.5ml$of acetone is taken in a flask. Powdered iodine is added to the flask, stir the mixture and place this mixture in a warm water bath.
Temperature is maintained at ${70^\circ }c$to ${80^\circ }c.$
When a solution is cooled yellow crystals of iodoform are obtained.
This method is an identification test for the presence of ketone groups in organic compounds.
This is also known as iodoform test.
Only methylated ketone shows this reaction.
If it means one of the alkyl groups in ketone should be methyl group.
So, the correct answer is “Option A”.
Additional Information:
Iodoform can also be prepared by ethanol. When ethyl alcohol is heated with iodine and sodium hydroxide or aqueous $N{a_2}C{O_3}$ form yellow crystalline solid iodoform.
$C{H_3}C{H_2}OH + C{I_2} + 6NaOH\xrightarrow{\Delta }CH{I_3} + HCOONa + 5NaI + 5{H_2}O$
Iodoform is a crystalline, volatile substance.
It has a distinctive odor and halogens to chloroform.
This is used as an antiseptic dressing.
Note:
As we discussed above only methylated ketones give iodoform test.
3-pentanone

Does not give iodoform test because it has two ethyl groups attached to carbonyl groups.
An aldehyde or ketone which has a methyl group attached to a carbonyl group will give a positive test.
Iodoform is prepared by ethyl alcohol and acetone in laboratories.
Complete step by step answer:
(1) Acetone is when treated with iodine and potassium hydroxide, producing iodoform.
When reaction takes place in following steps.
(2) In the first step iodine reacts with acetone in presence of $KOH$ and form tri-iodo-acetone.
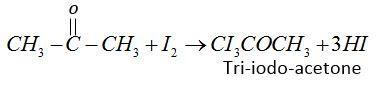
(3) Second step tri-iodo-ethane reacts with $KOH$and forms iodoform.
$C{I_3}COC{H_3} + KOH \to CH{I_3} + C{H_3}COOK$
Iodoform potassium acetate
Therefore, form the above explanation the correct option is (A) $KOH.$
About $3.5ml$of acetone is taken in a flask. Powdered iodine is added to the flask, stir the mixture and place this mixture in a warm water bath.
Temperature is maintained at ${70^\circ }c$to ${80^\circ }c.$
When a solution is cooled yellow crystals of iodoform are obtained.
This method is an identification test for the presence of ketone groups in organic compounds.
This is also known as iodoform test.
Only methylated ketone shows this reaction.
If it means one of the alkyl groups in ketone should be methyl group.
So, the correct answer is “Option A”.
Additional Information:
Iodoform can also be prepared by ethanol. When ethyl alcohol is heated with iodine and sodium hydroxide or aqueous $N{a_2}C{O_3}$ form yellow crystalline solid iodoform.
$C{H_3}C{H_2}OH + C{I_2} + 6NaOH\xrightarrow{\Delta }CH{I_3} + HCOONa + 5NaI + 5{H_2}O$
Iodoform is a crystalline, volatile substance.
It has a distinctive odor and halogens to chloroform.
This is used as an antiseptic dressing.
Note:
As we discussed above only methylated ketones give iodoform test.
3-pentanone

Does not give iodoform test because it has two ethyl groups attached to carbonyl groups.
An aldehyde or ketone which has a methyl group attached to a carbonyl group will give a positive test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




