
Acetyl nitrate has the structure:
A.
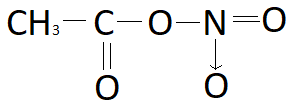
B.
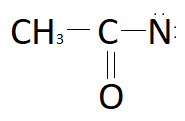
C.
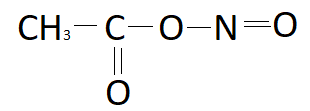
D.
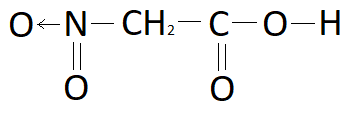
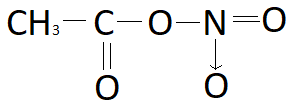
Answer
582k+ views
Hint: Acetic nitrate is an organic compound that is considered to be a mixed anhydride of nitric acid and acetic acid. It has a molecular formula \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C(O)ON}}{{\text{O}}_{\text{2}}}\]. Acetyl nitrate is a highly explosive and corrosive liquid. And the acid often fumes in moist air.
Complete step by step answer:
Acetic nitrate is made up of two chemical entities, one is acetate (${\text{C}}{{\text{H}}_{\text{3}}}{\text{ - CO}}$) and the other one is nitrate (Both of them are joined through a carbon-oxygen bond).
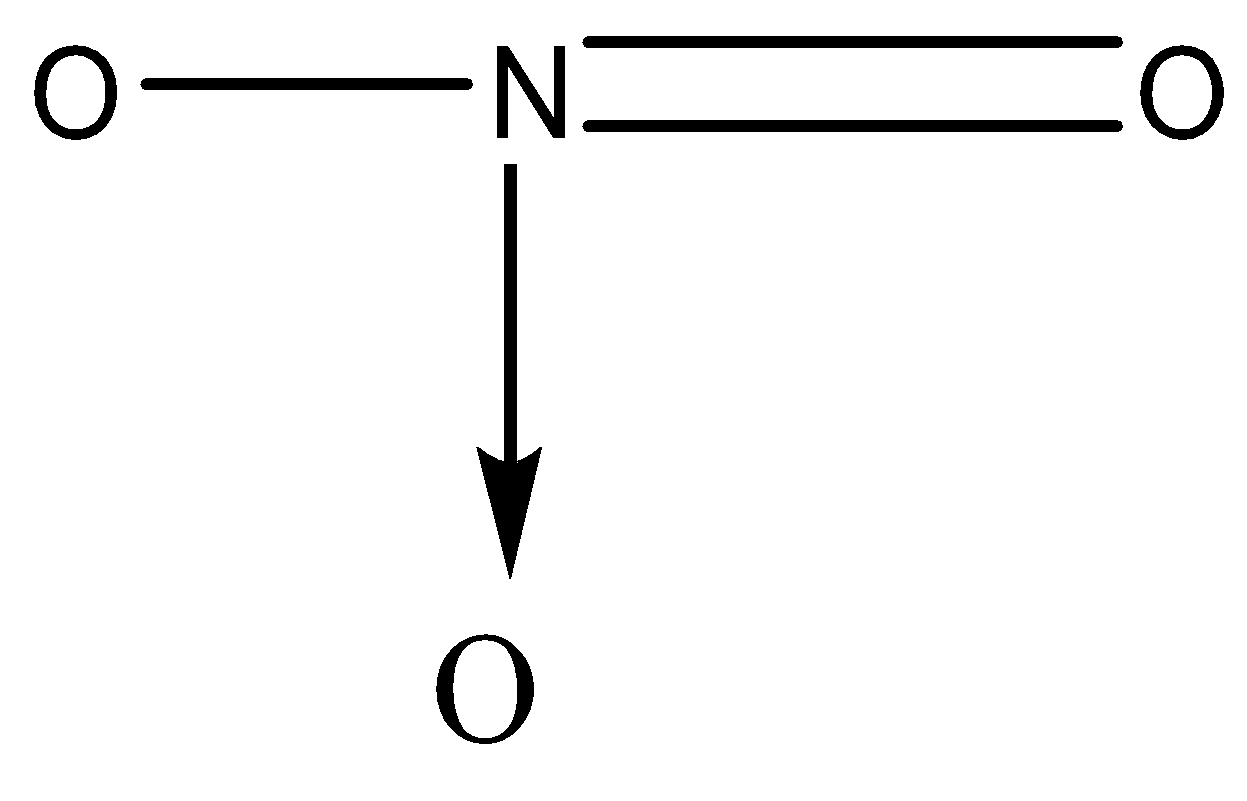
Both of them are joined through a carbon-oxygen bond.
And thus giving the following structure.
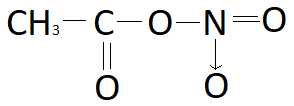
Preparation of acetic nitrate:
Acetic acid is prepared by using acetic anhydride and nitric acid. Sometimes, instead of nitric acid dinitrogen pentoxide is also used.
${{\text{(C}}{{\text{H}}_{\text{3}}}{\text{CO)}}_{\text{2}}}{\text{O + HN}}{{\text{O}}_{\text{3}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C(O)ON}}{{\text{O}}_{\text{2}}}{\text{ + C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$
Acetic nitrate when comes in contact with moist air it gets hydrolyzed to nitric acid and acetic acid.
So, the correct answer is Option C.
Note:
Some of the properties of acetic nitrate:
The compound occurs in a liquid state and has a boiling point of 22 degrees Celsius.
It is a colorless compound and has a molar mass of 105.05 g.
Acetic nitrate is highly explosive and thus is used in making explosives.
Acetic nitrate has a coordinate bond in its structure between nitrogen and oxygen while the rest of all bonds are covalent. A coordinate bond is a covalent bond in which both the electrons come from one atom. Here both the electrons are given by nitrogen. A coordinate bond is sometimes also referred to as a dative covalent bond. It is represented by an arrow between the atoms pointing towards the more electronegative one. The tail of the arrow is towards the atom who has given both the electrons.
Complete step by step answer:
Acetic nitrate is made up of two chemical entities, one is acetate (${\text{C}}{{\text{H}}_{\text{3}}}{\text{ - CO}}$) and the other one is nitrate (Both of them are joined through a carbon-oxygen bond).

Both of them are joined through a carbon-oxygen bond.
And thus giving the following structure.
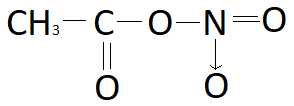
Preparation of acetic nitrate:
Acetic acid is prepared by using acetic anhydride and nitric acid. Sometimes, instead of nitric acid dinitrogen pentoxide is also used.
${{\text{(C}}{{\text{H}}_{\text{3}}}{\text{CO)}}_{\text{2}}}{\text{O + HN}}{{\text{O}}_{\text{3}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C(O)ON}}{{\text{O}}_{\text{2}}}{\text{ + C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$
Acetic nitrate when comes in contact with moist air it gets hydrolyzed to nitric acid and acetic acid.
So, the correct answer is Option C.
Note:
Some of the properties of acetic nitrate:
The compound occurs in a liquid state and has a boiling point of 22 degrees Celsius.
It is a colorless compound and has a molar mass of 105.05 g.
Acetic nitrate is highly explosive and thus is used in making explosives.
Acetic nitrate has a coordinate bond in its structure between nitrogen and oxygen while the rest of all bonds are covalent. A coordinate bond is a covalent bond in which both the electrons come from one atom. Here both the electrons are given by nitrogen. A coordinate bond is sometimes also referred to as a dative covalent bond. It is represented by an arrow between the atoms pointing towards the more electronegative one. The tail of the arrow is towards the atom who has given both the electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




