
A.Define optical active isomers
B.Write the conformational isomers of propane
Answer
578.1k+ views
Hint: Optical isomers are the two compounds which contain the same number and kinds of atoms and bonds i.e. the connectivity between atoms is same but they are non-superimposable mirror images. Moreover, conformational isomers are a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds.
Complete step by step answer:
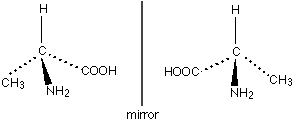
Basically, isomers are those compounds which have the same molecular formula but different bonding arrangement among atoms. Now, optical isomerism is a case where the isomers display identical characteristics in terms of molecular weight as well as chemical properties. This occurs mainly in substances that have the same molecular and structural formula but they cannot be superimposed on each other. We can say that they are mirror images of each other. An example is as shown:
Now, in the second option we have to write the conformational isomers of propane. Conformational isomers are the isomers having the same bond connectivity sequence and can be interconverted by rotation around one or more single i.e. sigma bonds. Further, they are of two types, one is eclipse conformation and other is staggered conformation. Now, let’s draw the conformational isomers of propane.
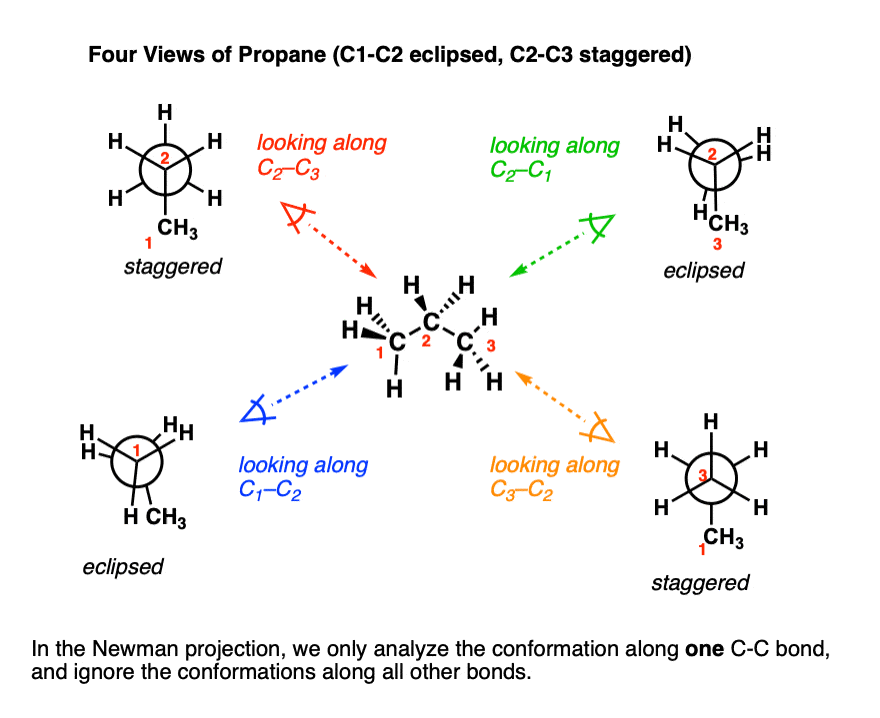
Note: The conformation in which the hydrogen atom is attached to two carbon atoms nearest to each other as possible is known as eclipsed. On the other hand, the conformation in which hydrogen atoms are attached to two carbons and are as far as possible with respect to each other is known as staggered conformation. This configuration is more stable as compared to eclipse conformation as there are minimum repulsive forces and minimum energy.
Complete step by step answer:
Basically, isomers are those compounds which have the same molecular formula but different bonding arrangement among atoms. Now, optical isomerism is a case where the isomers display identical characteristics in terms of molecular weight as well as chemical properties. This occurs mainly in substances that have the same molecular and structural formula but they cannot be superimposed on each other. We can say that they are mirror images of each other. An example is as shown:

Now, in the second option we have to write the conformational isomers of propane. Conformational isomers are the isomers having the same bond connectivity sequence and can be interconverted by rotation around one or more single i.e. sigma bonds. Further, they are of two types, one is eclipse conformation and other is staggered conformation. Now, let’s draw the conformational isomers of propane.

Note: The conformation in which the hydrogen atom is attached to two carbon atoms nearest to each other as possible is known as eclipsed. On the other hand, the conformation in which hydrogen atoms are attached to two carbons and are as far as possible with respect to each other is known as staggered conformation. This configuration is more stable as compared to eclipse conformation as there are minimum repulsive forces and minimum energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




