
Aluminium crystallizes in an FCC structure. Atomic radius of the metal is 125pm. What is the length of the side of the unit cell of the metal?
Answer
584.1k+ views
Hint: Face centered cubic [FCC] crystal structure has eight spheres at corner of cube and one sphere at center of each face of cube.
Formula used: $a = \sqrt 8 r$
Complete step by step answer: The FCC structure is given as:
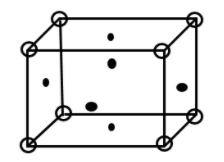
Given that aluminium crystallizes in FCC structure.
Therefore, we first calculate No. of molecules present in FCC structure.
8 molecules present at corner of cube therefore No. of molecule at corner$ = \dfrac{1}{8} \times 8$
6 molecules present on face of cube therefore No. of molecule at faces$ = \dfrac{1}{2} \times 6$ = 3
Total molecule = 3 + 1= 4
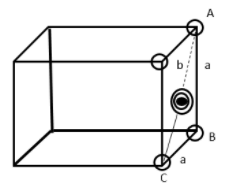
Consider triangle ABC of a side of a face centered cubic unit cell.
The length of edge = a
Hypotenuse AC = b
By Pythagoras theorem
$A{C^2} = A{B^2} + B{C^2}$
${b^2} = {a^2} + {a^2}$
${b^2} = 2{a^2}$ . . . . . (1)
Since $b = 4r$ $r = $radius of sphere.
Hypotenuse b has one sphere and two half spheres
Putting value of b in equation (1)
${\left( {4r} \right)^2} = 2{a^2}$
$16{r^2} = 2{a^2}$
${a^2} = \dfrac{{16{r^2}}}{2} = 8{r^2}$
$\therefore a = \sqrt 8 r$
In given problem $r = 125pm.$
So length of side of unit cell is
$a = \sqrt 8 \times 125$
$\sqrt 8 = \sqrt {4 \times 2} $
$ = 2\sqrt 2 $
$a = 2\sqrt 2 \times 125$
$\sqrt 2 = 1.414$
$a = 2 \times 1.414 \times 125$
a = 353.5pm
Therefore, the edge length of face centered cubic structure of aluminium is 353.5 pm.
Note: The relation between edge length of crystal structure and radius of sphere is different in different structures. This is as follows.
Simple cubic a = 2r
Face centered cubic[FCC] $a = \sqrt 8 r$
Body centered cubic[BCC] $a = \dfrac{{4r}}{{\sqrt 3 }}$
Formula used: $a = \sqrt 8 r$
Complete step by step answer: The FCC structure is given as:

Given that aluminium crystallizes in FCC structure.
Therefore, we first calculate No. of molecules present in FCC structure.
8 molecules present at corner of cube therefore No. of molecule at corner$ = \dfrac{1}{8} \times 8$
6 molecules present on face of cube therefore No. of molecule at faces$ = \dfrac{1}{2} \times 6$ = 3
Total molecule = 3 + 1= 4

Consider triangle ABC of a side of a face centered cubic unit cell.
The length of edge = a
Hypotenuse AC = b
By Pythagoras theorem
$A{C^2} = A{B^2} + B{C^2}$
${b^2} = {a^2} + {a^2}$
${b^2} = 2{a^2}$ . . . . . (1)
Since $b = 4r$ $r = $radius of sphere.
Hypotenuse b has one sphere and two half spheres
Putting value of b in equation (1)
${\left( {4r} \right)^2} = 2{a^2}$
$16{r^2} = 2{a^2}$
${a^2} = \dfrac{{16{r^2}}}{2} = 8{r^2}$
$\therefore a = \sqrt 8 r$
In given problem $r = 125pm.$
So length of side of unit cell is
$a = \sqrt 8 \times 125$
$\sqrt 8 = \sqrt {4 \times 2} $
$ = 2\sqrt 2 $
$a = 2\sqrt 2 \times 125$
$\sqrt 2 = 1.414$
$a = 2 \times 1.414 \times 125$
a = 353.5pm
Therefore, the edge length of face centered cubic structure of aluminium is 353.5 pm.
Note: The relation between edge length of crystal structure and radius of sphere is different in different structures. This is as follows.
Simple cubic a = 2r
Face centered cubic[FCC] $a = \sqrt 8 r$
Body centered cubic[BCC] $a = \dfrac{{4r}}{{\sqrt 3 }}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




