
When ammonia cyanate is heated for a long time, the product is:
(A) Nitrous oxide
(B) Ammonium cyanide
(C) Nitrogen and water
(D) Biuret
Answer
583.2k+ views
Hint: When ammonium cyanate is heated for a short time it produces urea which is the first organic compound prepared in the laboratory. When urea is heated the product formed is a chemical compound which is soluble in hot water.
Complete step by step solution:
Reaction of production of urea from ammonium cyanate:
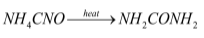
The reaction by which ammonium cyanate is converted to urea is Wohler synthesis. This reaction takes place in two steps: in the first step ammonium cyanate decomposes into ammonia and cyanic acid and in the second step the ammonia and cyanic acid reacts reversibly to form urea.
The reactions involved is mentioned below:
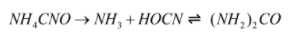
After the production of urea, it is heated further and biuret is produced.
The reaction involves is:
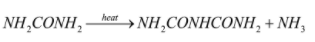
Biuret is a white solid which is soluble in hot water. It is used as a non-protein source in ruminant feed, and then it is converted to protein by the gut microorganisms.
Hence the correct answer is option (D).
Additional information:
Uses and properties of urea-
Urea is used as a fertilizer because the nitrogen content in urea is quite high.
Urea dissolves in the kidney and blood excretes it with urine.
Urea behaves as a weak monobasic acid.
In urea there is a carbonyl group which is attached to two amide groups. In the carbonyl group present oxygen atom is bonded to a double bonded carbon.
Note: To prevent the formation of biuret while heating ammonium cyanate the temperature must be kept just above the melting point. This is also accomplished by adding liquid ammonia to urea aqueous streams.
Complete step by step solution:
Reaction of production of urea from ammonium cyanate:

The reaction by which ammonium cyanate is converted to urea is Wohler synthesis. This reaction takes place in two steps: in the first step ammonium cyanate decomposes into ammonia and cyanic acid and in the second step the ammonia and cyanic acid reacts reversibly to form urea.
The reactions involved is mentioned below:
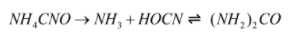
After the production of urea, it is heated further and biuret is produced.
The reaction involves is:
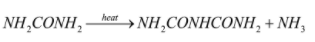
Biuret is a white solid which is soluble in hot water. It is used as a non-protein source in ruminant feed, and then it is converted to protein by the gut microorganisms.
Hence the correct answer is option (D).
Additional information:
Uses and properties of urea-
Urea is used as a fertilizer because the nitrogen content in urea is quite high.
Urea dissolves in the kidney and blood excretes it with urine.
Urea behaves as a weak monobasic acid.
In urea there is a carbonyl group which is attached to two amide groups. In the carbonyl group present oxygen atom is bonded to a double bonded carbon.
Note: To prevent the formation of biuret while heating ammonium cyanate the temperature must be kept just above the melting point. This is also accomplished by adding liquid ammonia to urea aqueous streams.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




