
An atom with atomic number 21 belongs to the category of:
(A) s- block elements
(B) p-block elements
(C) d- block elements
(D) f-block elements
Answer
588.6k+ views
Hint: Periodic table is a tabular display of the chemical elements, which are arranged by the atomic number, electronic configuration and chemical properties. Transition metals are the d-block elements of the periodic table and they belong to groups 3 to 12
Complete step by step answer:
The periodic table is an arrangement of all the elements in accordance with their increasing atomic number and recurring chemical properties. They are assorted in a tabular arrangement wherein a row is a period and a column is a group.
Now, let’s write the electronic configuration of the element with atomic number 21.
Electronic configuration$ = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^1}$ or $[Ar]3{d^1}4{s^2}$
Now, based on the electronic configuration we can see that the element has partially filled d subshell and hence, it belongs to d –block elements. Further, the name of the element is Scandium. You can see the element in the periodic table given below:
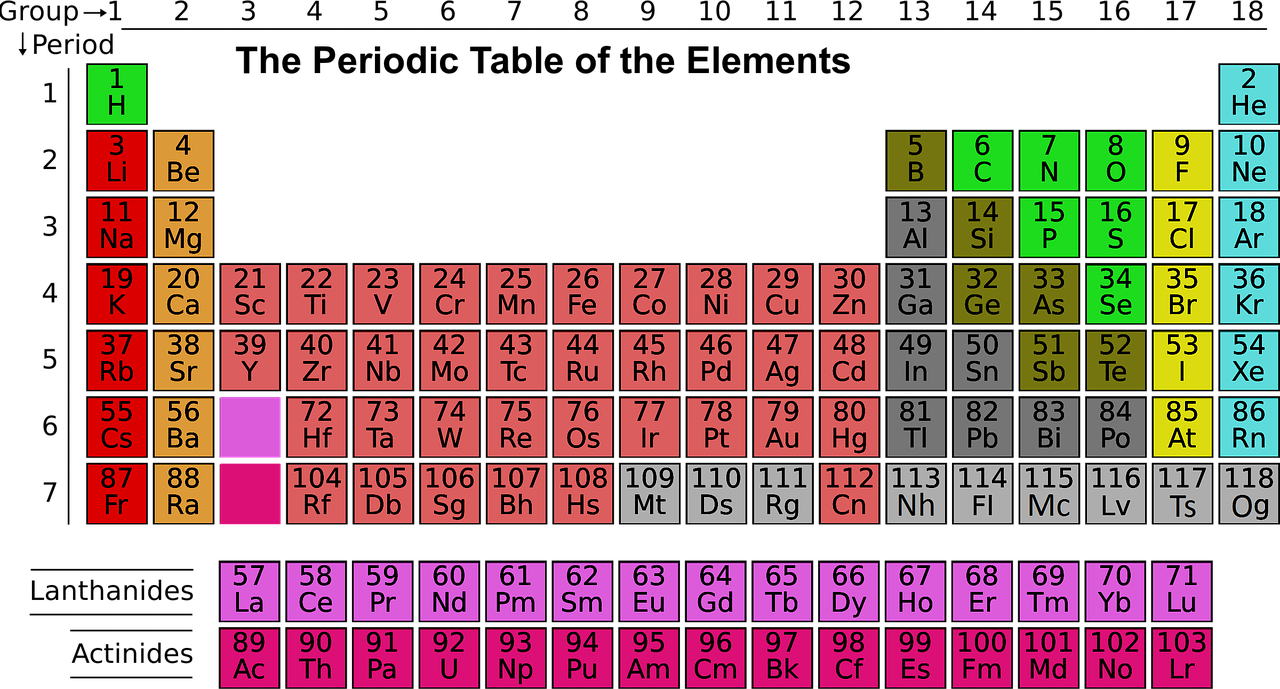
The d-block elements are the elements which can be found from the third group to the twelfth group of the modern periodic table. The valence electron falls in the d orbital. These are also known as transition elements or transition metals.
Hence, option C is correct.
Note: Scandium has a high melting point and low density and due to this fact, it is mainly used in the form of an alloying agent in the category of metals for application of high performance and military purposes. Apart from this, this metal is also used as an alloy additive to alloys that are aluminium based, for making metal halide lamps of high intensity and sporting goods.
Complete step by step answer:
The periodic table is an arrangement of all the elements in accordance with their increasing atomic number and recurring chemical properties. They are assorted in a tabular arrangement wherein a row is a period and a column is a group.
Now, let’s write the electronic configuration of the element with atomic number 21.
Electronic configuration$ = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^1}$ or $[Ar]3{d^1}4{s^2}$
Now, based on the electronic configuration we can see that the element has partially filled d subshell and hence, it belongs to d –block elements. Further, the name of the element is Scandium. You can see the element in the periodic table given below:

The d-block elements are the elements which can be found from the third group to the twelfth group of the modern periodic table. The valence electron falls in the d orbital. These are also known as transition elements or transition metals.
Hence, option C is correct.
Note: Scandium has a high melting point and low density and due to this fact, it is mainly used in the form of an alloying agent in the category of metals for application of high performance and military purposes. Apart from this, this metal is also used as an alloy additive to alloys that are aluminium based, for making metal halide lamps of high intensity and sporting goods.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




