
An element which is an essential constituent of all organic compounds belongs to the _______group.
(A) 5th
(B) 8th
(C) 14th
(D) 12th
Answer
579k+ views
Hint: Organic compounds are those compounds that contain carbon hydrogen bond. Carbon is essentially present in organic compounds.
Complete step by step answer:
Carbon is an essential element of organic compounds.
Atomic number of carbon is 6.
It’s electronic configuration is 2,4 or $1{s^2}2{s^2}2p_x^12p_y^12p_z^0$
Electronic configuration carbon atom at ground state
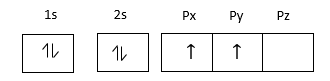
Electronic configuration carbon atom at excited state
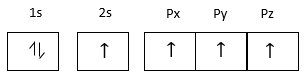
Carbon atom forms four covalent bonds with other atoms therefore its valiancy is four.
Carbon is a p-block element because the last electron enters in p-orbitals.
The group number can be predicted as
Group number$ = 10 + $Numbers of electron in valence shell
$ = 10 + 4$
$ = {14^{th}}$ group.
Therefore, from the above explanation, the correct option is (C) 14th group.
Additional Information:
Organic compounds make up the bulk of living organisms. There are four categories that are found in living things.
They are carbohydrates, lipids, proteins and nucleic Acid.
All these compounds contain C-element as an essential element.
Organic compounds found throughout the world, in soils and seas, commercial products and every cell of the human body.
Carbon atoms have the unique property of catenation. Catenation is the bonding of atoms of the same element into a series called chain. They are present either in the form of chain, branch or a ring. Therefore, it forms millions of organic compounds. Carbon shows the property of catenation to the maximum extent.
Note:
Carbon has very unique properties.
They are- It is abundant and forms strong covalent bonds, it has four valence electrons, it has a variety of shapes, and it bonds with multiple elements.
Complete step by step answer:
Carbon is an essential element of organic compounds.
Atomic number of carbon is 6.
It’s electronic configuration is 2,4 or $1{s^2}2{s^2}2p_x^12p_y^12p_z^0$
Electronic configuration carbon atom at ground state

Electronic configuration carbon atom at excited state

Carbon atom forms four covalent bonds with other atoms therefore its valiancy is four.
Carbon is a p-block element because the last electron enters in p-orbitals.
The group number can be predicted as
Group number$ = 10 + $Numbers of electron in valence shell
$ = 10 + 4$
$ = {14^{th}}$ group.
Therefore, from the above explanation, the correct option is (C) 14th group.
Additional Information:
Organic compounds make up the bulk of living organisms. There are four categories that are found in living things.
They are carbohydrates, lipids, proteins and nucleic Acid.
All these compounds contain C-element as an essential element.
Organic compounds found throughout the world, in soils and seas, commercial products and every cell of the human body.
Carbon atoms have the unique property of catenation. Catenation is the bonding of atoms of the same element into a series called chain. They are present either in the form of chain, branch or a ring. Therefore, it forms millions of organic compounds. Carbon shows the property of catenation to the maximum extent.
Note:
Carbon has very unique properties.
They are- It is abundant and forms strong covalent bonds, it has four valence electrons, it has a variety of shapes, and it bonds with multiple elements.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




