
An example of double salt is:
A. potash alum
B. hypo
C. ${{\text{K}}_{4}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }$
D. bleaching powder
Answer
596.1k+ views
Hint: A double salt is a crystalline salt which is the composition of a mixture of two simple salts but with a different crystal structure. Double salts are ionisable when dissolved in water.
Complete step by step answer:
-A double salt is a compound composed of the mixture of two different salts compounds. For example: Mohr’s salt
-Now let us get more briefing of the options:
(A) Potash alum: It has other names also like potassium alum or potassium aluminium sulphate. It is a chemical compound with chemical formula$\text{KAl(S}{{\text{O}}_{4}}{{\text{)}}_{2}}\text{.12}{{\text{H}}_{2}}\text{O}$. It comes under the category of hydrated double sulphate salt as it ionizes in water. Potash alum is prepared by dissolving hydrated aluminium sulphate and potassium sulphate in equal amounts in water having sulphuric acid. This is how; alum separates out from the mixture.
(B) Hypo: It is an inorganic salt with various names as Sodium thiosulfate or sodium hyposulphite. The chemical formula of hypo
 is$\text{N}{{\text{a}}_{2}}{{\text{S}}_{2}}{{\text{O}}_{3}}$and molar mass is 158 grams. It is not a double salt. It is used in iodometric titration to determine concentration of reductant.
is$\text{N}{{\text{a}}_{2}}{{\text{S}}_{2}}{{\text{O}}_{3}}$and molar mass is 158 grams. It is not a double salt. It is used in iodometric titration to determine concentration of reductant.
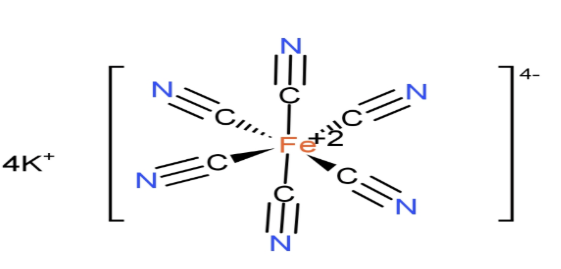
(C)${{\text{K}}_{4}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }$: It is inorganic compound potassium salt with names like Potassium ferrocyanide or potassium hexacyanoferrate. The molar mass of ${{\text{K}}_{4}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }$is 422 grams. It is potassium salt of coordination complex${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{4-}}$.
(D) Bleaching powder: It is an inorganic compound with chemical name as Calcium hypochlorite and chemical formula is$\text{Ca(ClO}{{\text{)}}_{2}}$. Other names of the compound are chlorine powder, chlorinated lime which is used in water treatment plants. The molar mass of the compound is 143 grams. Bleaching powder is a mixed salt as it contains 1 cation ($\text{C}{{\text{a}}^{+2}}$) and 2 anions ($\text{C}{{\text{l}}^{-}}$) and ($\text{OC}{{\text{l}}^{-}}$).
-The correct answer is potash alum which is option A.
Additional Information:
Uses of potash alum:
(1) The use of potash alum for textiles, wood and paperless flame resistance is as fire-retardant.
(2) For leather tanning, it is used to extract moisture from the hide and avoid rotting. Potash alum is not covered and can be washed out, as compared to tannic acid.
(3) Alum was used to form a link between natural textile fibres like wool and dye, as mordant.
Note: The double salt should not be confused with complex salts. There is a difference between the two terms.
Complete step by step answer:
-A double salt is a compound composed of the mixture of two different salts compounds. For example: Mohr’s salt
-Now let us get more briefing of the options:
(A) Potash alum: It has other names also like potassium alum or potassium aluminium sulphate. It is a chemical compound with chemical formula$\text{KAl(S}{{\text{O}}_{4}}{{\text{)}}_{2}}\text{.12}{{\text{H}}_{2}}\text{O}$. It comes under the category of hydrated double sulphate salt as it ionizes in water. Potash alum is prepared by dissolving hydrated aluminium sulphate and potassium sulphate in equal amounts in water having sulphuric acid. This is how; alum separates out from the mixture.
(B) Hypo: It is an inorganic salt with various names as Sodium thiosulfate or sodium hyposulphite. The chemical formula of hypo

(C)${{\text{K}}_{4}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }$: It is inorganic compound potassium salt with names like Potassium ferrocyanide or potassium hexacyanoferrate. The molar mass of ${{\text{K}}_{4}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }$is 422 grams. It is potassium salt of coordination complex${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{4-}}$.
(D) Bleaching powder: It is an inorganic compound with chemical name as Calcium hypochlorite and chemical formula is$\text{Ca(ClO}{{\text{)}}_{2}}$. Other names of the compound are chlorine powder, chlorinated lime which is used in water treatment plants. The molar mass of the compound is 143 grams. Bleaching powder is a mixed salt as it contains 1 cation ($\text{C}{{\text{a}}^{+2}}$) and 2 anions ($\text{C}{{\text{l}}^{-}}$) and ($\text{OC}{{\text{l}}^{-}}$).
-The correct answer is potash alum which is option A.
Additional Information:
Uses of potash alum:
(1) The use of potash alum for textiles, wood and paperless flame resistance is as fire-retardant.
(2) For leather tanning, it is used to extract moisture from the hide and avoid rotting. Potash alum is not covered and can be washed out, as compared to tannic acid.
(3) Alum was used to form a link between natural textile fibres like wool and dye, as mordant.
Note: The double salt should not be confused with complex salts. There is a difference between the two terms.
| DOUBLE SALT | COMPLEX SALT |
| A double salt is prepared by the combination of two different salts components. | A complex salt is composed of a central metal atom having coordination bonds with ligands attached to it. |
| Give simple ions when added to water. | Do not give simple ions. |
| Gets completely dissociated into ions when dissolved in water. | Do not completely dissociate into ions. |
| Can be easily analyzed by determining the ions present in aqueous solution. | Cannot be easily analyzed by determining the ions present in aqueous solution. |
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




