
An important reaction of acetone is auto condensation in presence of concentrated sulphuric acid to give the aromatic compound _______
A. Mesitylene
B. Mesityl oxide
C. Trioxane
D. Phorone
Answer
582.6k+ views
Hint: Consider the mechanism of an acid catalysed aldol condensation reaction which involves the conversion of ketone or aldehyde to an enol, which then attacks another aldehyde or ketone which has been activated by protonation of the carbonyl oxygen and further forming a carbon-carbon structure.
Complete step by step solution:
In the given question, given that acetone which is a ketone which undergoes auto condensation i.e. self-reaction in the presence of concentrated sulphuric acid and then, an aromatic compound is formed. So, this reaction is called acid catalysed aldol condensation.
So, first of all let us know what an acid catalysed aldol condensation process is.
The acid catalysed aldol condensation reaction is an additional reaction between an enol and an aldehyde or ketone. It is usually used to join two identical aldehydes or ketones together (which is called dimerization), or to join two different aldehydes or ketones (this is called cross aldol).
The process begins with the conversion of a ketone or an aldehyde to an enol first, which then attacks another aldehyde group or ketone group (which has been activated by the protonation of the carbonyl oxygen) to form carbon-carbon structures.
So, here let us consider three molecules of acetone will undergo auto condensation in the presence of concentrated sulphuric acid, which will further eliminate three molecules of water and forming a product $1,3,5-TrimethylBenzene$, which is commonly called as “Mesitylene”. So, the reaction will look like as shown below:
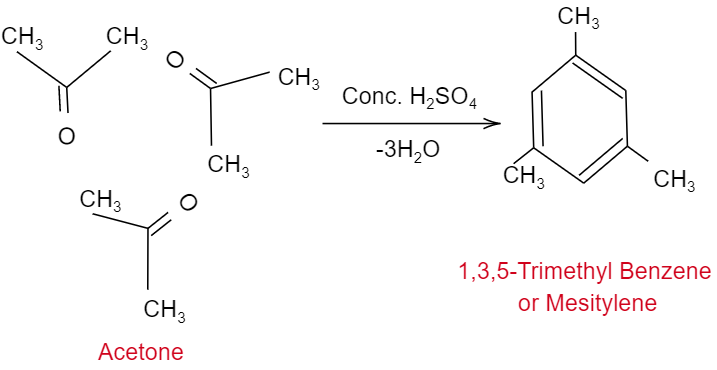
Hence, the correct option is A.
Note: Remember, when acetone saturated with hydrochloride gas is kept at low temperature for a few days, it forms mesityl oxide and phorone which can be further separated by distillation. Acetone when is condensed in the presence of a dilute base, it will tend to form di acetone alcohol or aldol and when diacetone is heated in the presence of little amount of iodine, mesityl oxide is formed.
Complete step by step solution:
In the given question, given that acetone which is a ketone which undergoes auto condensation i.e. self-reaction in the presence of concentrated sulphuric acid and then, an aromatic compound is formed. So, this reaction is called acid catalysed aldol condensation.
So, first of all let us know what an acid catalysed aldol condensation process is.
The acid catalysed aldol condensation reaction is an additional reaction between an enol and an aldehyde or ketone. It is usually used to join two identical aldehydes or ketones together (which is called dimerization), or to join two different aldehydes or ketones (this is called cross aldol).
The process begins with the conversion of a ketone or an aldehyde to an enol first, which then attacks another aldehyde group or ketone group (which has been activated by the protonation of the carbonyl oxygen) to form carbon-carbon structures.
So, here let us consider three molecules of acetone will undergo auto condensation in the presence of concentrated sulphuric acid, which will further eliminate three molecules of water and forming a product $1,3,5-TrimethylBenzene$, which is commonly called as “Mesitylene”. So, the reaction will look like as shown below:

Hence, the correct option is A.
Note: Remember, when acetone saturated with hydrochloride gas is kept at low temperature for a few days, it forms mesityl oxide and phorone which can be further separated by distillation. Acetone when is condensed in the presence of a dilute base, it will tend to form di acetone alcohol or aldol and when diacetone is heated in the presence of little amount of iodine, mesityl oxide is formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




