
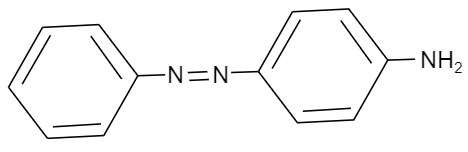
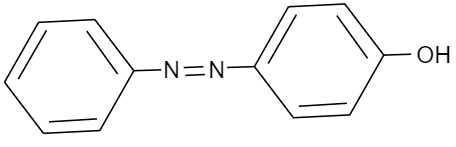
Aniline dissolved in dilute $HCl$ is reacted with sodium nitrite at ${{0}^{o}}C$. This solution was added dropwise to a solution containing an equimolar mixture of aniline and phenol in dil. $HCl$. The structure of the major product is:
(A)
(B)
(C)
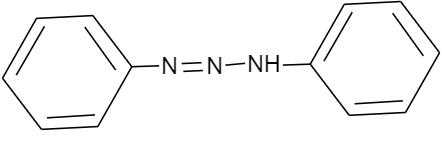
(D)
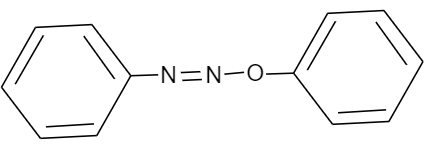


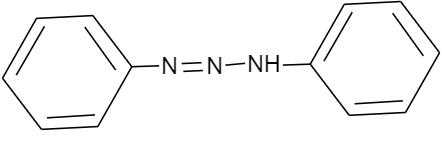
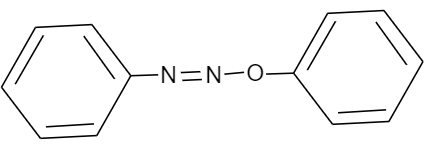
Answer
577.5k+ views
Hint: You should know that when a primary aromatic amine which is dissolved in dilute acid is treated with sodium nitrite, it will undergo diazotization process, where a diazonium salt is formed and further when this diazonium salt is treated with phenol and aniline, diazo coupling will occur.
Complete step by step solution:
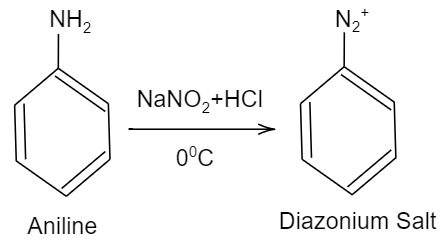
Given that, Aniline is being dissolved in dilute $HCl$ and is reacted with sodium nitrite at ${{0}^{o}}C$. Then the solution formed after the reaction, was added dropwise to a solution containing equimolar mixture of aniline and phenol in dil. $HCl$. So, we should know that aniline is a primary aromatic amine with chemical formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$. And we know that, when a primary aromatic amine when dissolved in dilute strong acid like hydrochloric acid and is reacted with nitrous acid such as sodium nitrite ($NaN{{O}_{2}}$), it will undergo diazotization forming a diazonium salt in the product. This reaction can be shown as follows:
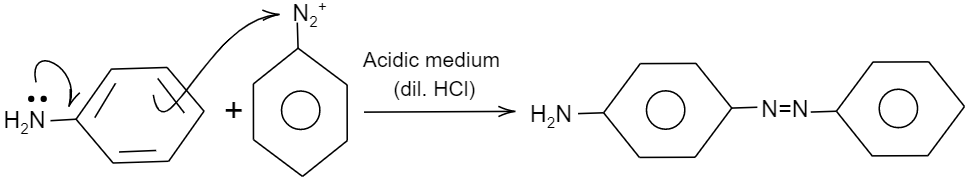
Further as per the given question, the solution containing diazonium salt is added to a solution containing phenol (${{C}_{6}}{{H}_{5}}OH$) and aniline in acidic medium. As we know in diazonium salt, the nitrogen present is electron deficient and requires electrons to be stable. So, it will require a group which should be electron rich, so that it can donate to nitrogen of the diazonium salt. Therefore, by comparing phenol and aniline, aniline is electron rich. So, aniline will attack the nitrogen group of the diazonium salt forming a double bond. This reaction is shown as follows:
Hence, the correct option is A.
Note: It is important to note that aniline is electron rich than phenol because the oxygen atom present in the hydroxyl group of phenol is more electronegative than the nitrogen atom present in the amine group of aniline. So, when diazonium salt is treated with these two components, aniline will attack on the salt as it is electron rich and the salt is electron deficient.
Complete step by step solution:
Given that, Aniline is being dissolved in dilute $HCl$ and is reacted with sodium nitrite at ${{0}^{o}}C$. Then the solution formed after the reaction, was added dropwise to a solution containing equimolar mixture of aniline and phenol in dil. $HCl$. So, we should know that aniline is a primary aromatic amine with chemical formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$. And we know that, when a primary aromatic amine when dissolved in dilute strong acid like hydrochloric acid and is reacted with nitrous acid such as sodium nitrite ($NaN{{O}_{2}}$), it will undergo diazotization forming a diazonium salt in the product. This reaction can be shown as follows:

Further as per the given question, the solution containing diazonium salt is added to a solution containing phenol (${{C}_{6}}{{H}_{5}}OH$) and aniline in acidic medium. As we know in diazonium salt, the nitrogen present is electron deficient and requires electrons to be stable. So, it will require a group which should be electron rich, so that it can donate to nitrogen of the diazonium salt. Therefore, by comparing phenol and aniline, aniline is electron rich. So, aniline will attack the nitrogen group of the diazonium salt forming a double bond. This reaction is shown as follows:

Hence, the correct option is A.
Note: It is important to note that aniline is electron rich than phenol because the oxygen atom present in the hydroxyl group of phenol is more electronegative than the nitrogen atom present in the amine group of aniline. So, when diazonium salt is treated with these two components, aniline will attack on the salt as it is electron rich and the salt is electron deficient.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




