
What is the approximate wavelength of blue light?
$\begin{align}
& A.800A{}^\circ \\
& B.1600A{}^\circ \\
& C.3200A{}^\circ \\
& D.4800A{}^\circ \\
\end{align}$
Answer
548.1k+ views
Hint: The visible white light we see is a spectrum of seven magnificent colours. The seven colours, namely Violet, Indigo, Blue, Green, Yellow, Orange, and Red. The visible light is a part of the electromagnetic spectrum. The electromagnetic spectrum consists of a range of frequency ranging from a few Hertz to ${{10}^{25}}$ Hertz.
Complete step-by-step solution:
The human eye can view lights only of a certain wavelength, one of which being the visible light part of the electromagnetic spectrum whose wavelength ranges between \[380\times {{10}^{-9}}m\]to\[750\times {{10}^{-9}}m\].
We will see the wavelength ranges of different colours of the electromagnetic spectrum:
1) The wavelength of violet light ranges from \[380\times {{10}^{-9}}m\] to \[450\times {{10}^{-9}}m\].
2) The wavelength of blue light ranges from \[450\times {{10}^{-9}}m\] to \[495\times {{10}^{-9}}m\].
3) The wavelength of green light is \[495\times {{10}^{-9}}m\] to \[570\times {{10}^{-9}}m\].
4) The wavelength of yellow is \[570\times {{10}^{-9}}m\] to \[590\times {{10}^{-9}}m\].
5) The wavelength of orange light is \[590\times {{10}^{-9}}m\] to \[620\times {{10}^{-9}}m\].
6) The wavelength of red light is \[620\times {{10}^{-9}}m\] to \[750\times {{10}^{-9}}m\].
When we look at the above options, blue light has wavelength ranging from \[4500{{A}^{0}}-4950{{A}^{0}}\]
Hence, option D is the correct answer.
Note: The electromagnetic spectrum all together is a band consisting of Gamma radiation, X-ray radiation, Ultraviolet radiation, visible light, infrared radiation, Microwave radiation, Radio waves.
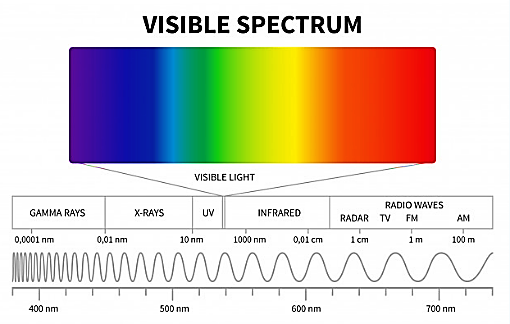
In the electromagnetic wave, a lower wavelength or higher frequency wave will have more energy. Therefore, gamma rays have the most energy and radio waves having the least energy. Electromagnetic waves interact with matter via their electric and magnetic fields which set in oscillation charges present in all matter. The detailed interaction and so the mechanism of absorption, scattering, etc., depend on the wavelength of the electromagnetic wave, and the nature of the atoms and molecules in the medium.
Complete step-by-step solution:
The human eye can view lights only of a certain wavelength, one of which being the visible light part of the electromagnetic spectrum whose wavelength ranges between \[380\times {{10}^{-9}}m\]to\[750\times {{10}^{-9}}m\].
We will see the wavelength ranges of different colours of the electromagnetic spectrum:
1) The wavelength of violet light ranges from \[380\times {{10}^{-9}}m\] to \[450\times {{10}^{-9}}m\].
2) The wavelength of blue light ranges from \[450\times {{10}^{-9}}m\] to \[495\times {{10}^{-9}}m\].
3) The wavelength of green light is \[495\times {{10}^{-9}}m\] to \[570\times {{10}^{-9}}m\].
4) The wavelength of yellow is \[570\times {{10}^{-9}}m\] to \[590\times {{10}^{-9}}m\].
5) The wavelength of orange light is \[590\times {{10}^{-9}}m\] to \[620\times {{10}^{-9}}m\].
6) The wavelength of red light is \[620\times {{10}^{-9}}m\] to \[750\times {{10}^{-9}}m\].
When we look at the above options, blue light has wavelength ranging from \[4500{{A}^{0}}-4950{{A}^{0}}\]
Hence, option D is the correct answer.
Note: The electromagnetic spectrum all together is a band consisting of Gamma radiation, X-ray radiation, Ultraviolet radiation, visible light, infrared radiation, Microwave radiation, Radio waves.

In the electromagnetic wave, a lower wavelength or higher frequency wave will have more energy. Therefore, gamma rays have the most energy and radio waves having the least energy. Electromagnetic waves interact with matter via their electric and magnetic fields which set in oscillation charges present in all matter. The detailed interaction and so the mechanism of absorption, scattering, etc., depend on the wavelength of the electromagnetic wave, and the nature of the atoms and molecules in the medium.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




