
Why are aliphatic amines stronger bases than aromatic amines?
Answer
529.7k+ views
Hint: Aliphatic amines are the amines which contain only H and alkyl substitutes like in dimethylamine whereas aromatic amines are the amines which have a nitrogen atom connected to an aromatic ring like in diphenylamine.
Complete answer step by step:
The hydrogen attached or the alkyl groups are electron donating groups. And we know that the stronger the electron donating group, the higher the +I effect and stronger is the base. Therefore, in aliphatic amines the $-N{H}_{2}$ group is attached to an electron donating alkyl group and thus show +I effect. And due to this reason the lone pair of electrons on nitrogen are easily available.
Whereas, the aromatic groups are known to be the electron withdrawing group, which means that they have lower basic strength in comparison with the alkyl groups. Therefore, in aromatic amines, the $-N{H}_{2}$ group is attached to the electron withdrawing $-{C}_{6}{H}_{5}$ group. Due to this the lone pair of electrons on nitrogen is not easily available.
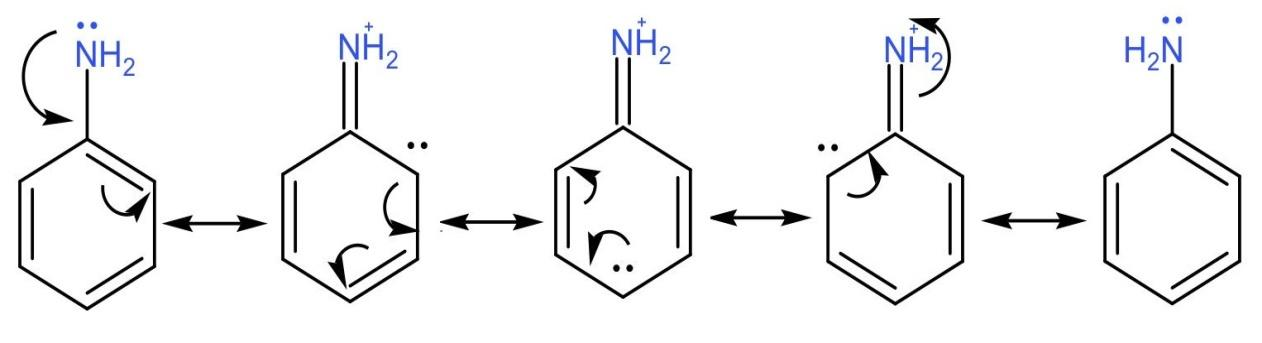
One more reason that aliphatic amines are a stronger base than aromatic amines is that the lone pair of electrons on nitrogen is in resonance in the aromatic ring and hence is available to the nitrogen atom itself.
Note: Aliphatic amines can also have aromatic rings but the nitrogen in aliphatic amines should be directly attached to the carbon atom of the alkyl group. Also the aliphatic heterocyclic amines are stronger than aromatic heterocyclic amines.
Complete answer step by step:
The hydrogen attached or the alkyl groups are electron donating groups. And we know that the stronger the electron donating group, the higher the +I effect and stronger is the base. Therefore, in aliphatic amines the $-N{H}_{2}$ group is attached to an electron donating alkyl group and thus show +I effect. And due to this reason the lone pair of electrons on nitrogen are easily available.
Whereas, the aromatic groups are known to be the electron withdrawing group, which means that they have lower basic strength in comparison with the alkyl groups. Therefore, in aromatic amines, the $-N{H}_{2}$ group is attached to the electron withdrawing $-{C}_{6}{H}_{5}$ group. Due to this the lone pair of electrons on nitrogen is not easily available.

One more reason that aliphatic amines are a stronger base than aromatic amines is that the lone pair of electrons on nitrogen is in resonance in the aromatic ring and hence is available to the nitrogen atom itself.
Note: Aliphatic amines can also have aromatic rings but the nitrogen in aliphatic amines should be directly attached to the carbon atom of the alkyl group. Also the aliphatic heterocyclic amines are stronger than aromatic heterocyclic amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




