
Are \[{N_2}\] and ${N_2}^ + $ paramagnetic or diamagnetic? Which one has the stronger bond?
Answer
495.6k+ views
Hint: To solve this question we should know about magnetic behavior of the elements, about their Molecular Orbital Diagram. Here in this question we have to draw the molecular orbital diagram of ${N_2}$ and ${N_2}^ + $ by which we can find the number of unpaired electrons. Hence with help of it, we can classify their magnetic nature.
Complete answer:
Paramagnetic substances are weakly influenced by the external magnetic field. They contain unpaired electrons in their orbitals. Paramagnetic substances have permanent dipole moments but with removal of magnetic field, the substance starts losing its magnetism.
Whereas, diamagnetic substances are those which do not get affected by external magnetic fields. They contain paired electrons in their orbitals. Diamagnetic substances show magnetization in the direction opposite to the magnetic field.
To find the magnetic behavior of the compound ${N_2}$ , we have to draw the molecular orbital diagram of ${N_2}$ by which we can get the number of unpaired electrons or paired electrons. As we know if a compound has unpaired electrons then that compound is paramagnetic in nature where if it contains paired electrons only then it is diamagnetic in nature.
The molecular orbital diagram of ${N_2}$ :
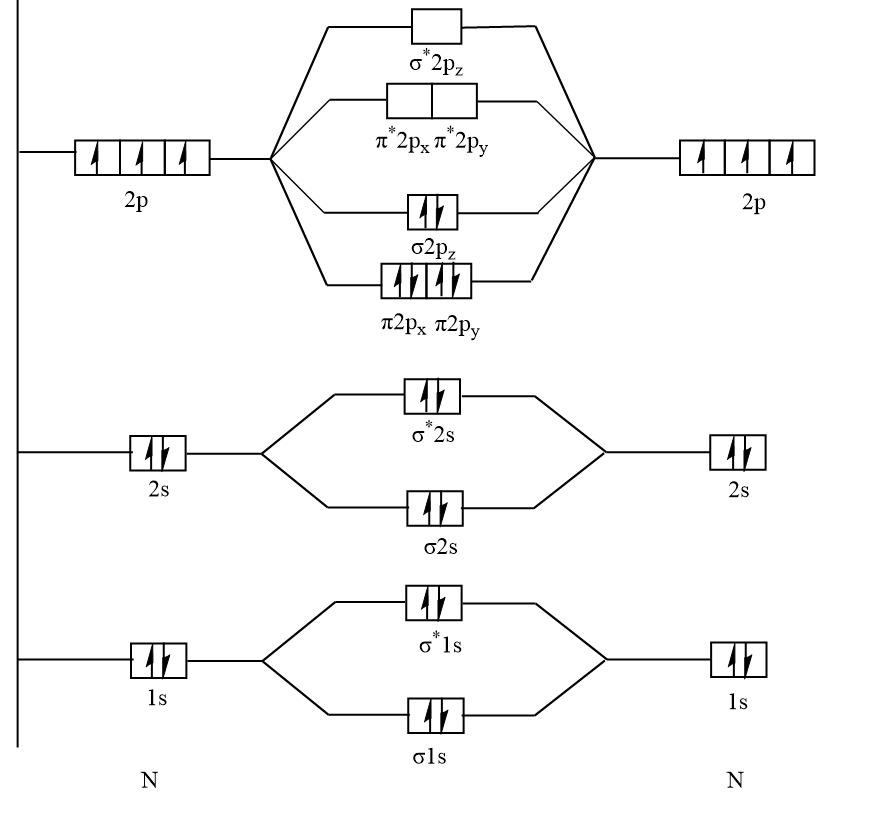
Here, with the help of a diagram it is clear that ${N_2}$ has no unpaired electrons that means ${N_2}$ is diamagnetic.
Again, to find the magnetic behavior of the compound ${N_2}^ + $ , we have to draw the molecular orbital diagram of ${N_2}^ + $ by which we can get a number of unpaired electrons or paired electrons.
The molecular orbital diagram of ${N_2}^ + $ :
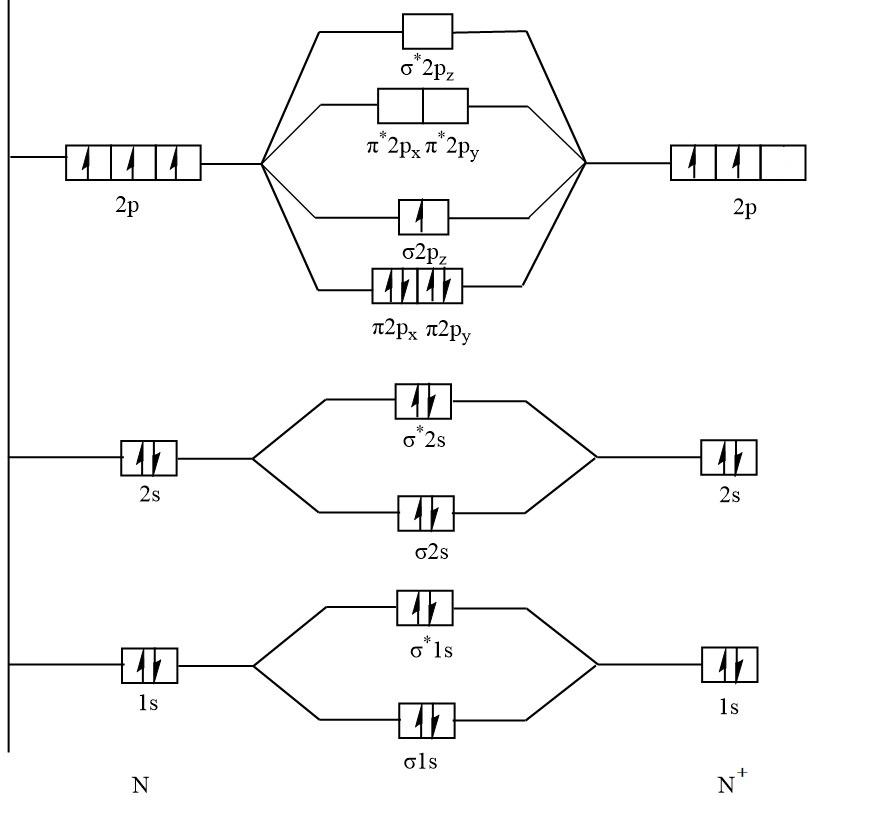
Again, with the help of a diagram it is clear that ${N_2}^ + $ has unpaired electrons in $\sigma 2{p_z}$ that means ${N_2}^ + $ is paramagnetic.
Further, we know that after losing an electron in a bonding molecular orbital; the bond becomes less strong as it is less thermodynamically stable hence, ${N_2}^ + $ has less bonding character than ${N_2}$.
Note:
Remember compounds having electrons less than or equal to 14 have negligible $s - p$ mixing and hence they follow normal pattern whereas compounds having more the 14 electrons have $s - p$ mixing, leads to the pattern in which ${\sigma _p}$ orbital is raised above the ${\pi _p}$ set.
Complete answer:
Paramagnetic substances are weakly influenced by the external magnetic field. They contain unpaired electrons in their orbitals. Paramagnetic substances have permanent dipole moments but with removal of magnetic field, the substance starts losing its magnetism.
Whereas, diamagnetic substances are those which do not get affected by external magnetic fields. They contain paired electrons in their orbitals. Diamagnetic substances show magnetization in the direction opposite to the magnetic field.
To find the magnetic behavior of the compound ${N_2}$ , we have to draw the molecular orbital diagram of ${N_2}$ by which we can get the number of unpaired electrons or paired electrons. As we know if a compound has unpaired electrons then that compound is paramagnetic in nature where if it contains paired electrons only then it is diamagnetic in nature.
The molecular orbital diagram of ${N_2}$ :

Here, with the help of a diagram it is clear that ${N_2}$ has no unpaired electrons that means ${N_2}$ is diamagnetic.
Again, to find the magnetic behavior of the compound ${N_2}^ + $ , we have to draw the molecular orbital diagram of ${N_2}^ + $ by which we can get a number of unpaired electrons or paired electrons.
The molecular orbital diagram of ${N_2}^ + $ :

Again, with the help of a diagram it is clear that ${N_2}^ + $ has unpaired electrons in $\sigma 2{p_z}$ that means ${N_2}^ + $ is paramagnetic.
Further, we know that after losing an electron in a bonding molecular orbital; the bond becomes less strong as it is less thermodynamically stable hence, ${N_2}^ + $ has less bonding character than ${N_2}$.
Note:
Remember compounds having electrons less than or equal to 14 have negligible $s - p$ mixing and hence they follow normal pattern whereas compounds having more the 14 electrons have $s - p$ mixing, leads to the pattern in which ${\sigma _p}$ orbital is raised above the ${\pi _p}$ set.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




