
What are the different types of adsorption? Give any three differences between characteristics of these different types of adsorption.
Answer
598.2k+ views
Hint: Adsorption is a surface phenomenon. Based on whether a chemical bond forms between adsorbate and adsorbent, it is mainly divided into two types.
Complete step by step answer:
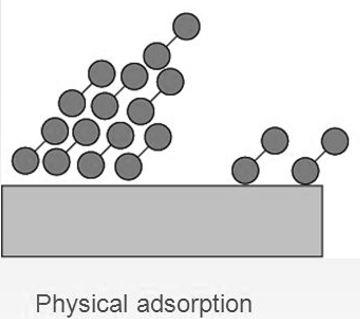
When a gas is adsorbed on the surface of a solid without the formation of any chemical bond between the adsorbate and the adsorbent it is called physisorption. It occurs due to weak Van der Waals forces between the two substances.
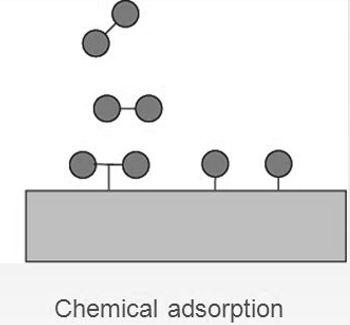
When a chemical bond forms between adsorbate and adsorbent the process is called Chemical adsorption or chemisorption.
Differences in their few characteristics are –
Note: Adsorption is a process which involves the accumulation of a substance in molecular species in higher concentration on the surface. Substance which is deposited on the surface of another substance is called adsorbate and Surface of a substance on which adsorbate accumulates is called adsorbent.
Complete step by step answer:
When a gas is adsorbed on the surface of a solid without the formation of any chemical bond between the adsorbate and the adsorbent it is called physisorption. It occurs due to weak Van der Waals forces between the two substances.
When a chemical bond forms between adsorbate and adsorbent the process is called Chemical adsorption or chemisorption.
Differences in their few characteristics are –
| Physisorption | Chemisorption |
| Occurs due to formation of weak Van der Waals forces between adsorbate and adsorbent. | Occurs due to strong chemical bonds between adsorbate and adsorbent. |
| It is a multi-layered process and is weak in nature. | It is a single-layered phenomenon but it is very strong in nature. |
| By increasing the temperature or by decreasing the pressure, the effects can be reversed. | If temperature is increased or pressure is decreased different compounds can be obtained but original ones cannot be restored i.e. it is irreversible. |
| It does not require any force for initiation of the process i.e. no activation energy is needed. | For initiation of the process, some energy is required i.e. it needs activation energy. |
| Physical adsorption is not specific and takes place all over the adsorbent. | Chemisorption is highly specific and takes place at reaction centres on the adsorbent. |
| 
|
Note: Adsorption is a process which involves the accumulation of a substance in molecular species in higher concentration on the surface. Substance which is deposited on the surface of another substance is called adsorbate and Surface of a substance on which adsorbate accumulates is called adsorbent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




