
What are the physical properties of liquids?
Answer
495.6k+ views
Hint: Liquids are mostly incompressible. They have an intermediate phase between solid and gas. The particles of solids are held together by strong intermolecular force whereas liquids are held together by intermolecular force which is weaker than that of solids.
Complete answer:
Liquid is mostly incompressible fluid that upholds the shape of its container. In liquids, molecules are closely packed. Like gas, there molecules do not have lots of space between them. So, they do not disperse to fill every space of a container like gas and have equitably constant density. They are made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds.
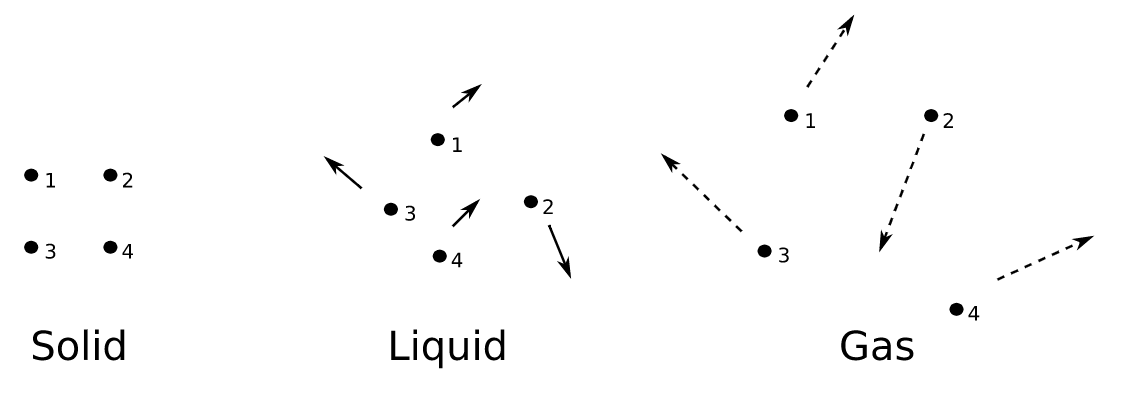
Liquids flow because the intermolecular forces between their molecules are weak enough to allow the molecules to move relative to one another. Intermolecular forces are the forces between two adjacent molecules.
Liquids have definite volume but indefinite shape. As the intermolecular attraction between the molecules of liquids are weaker than that of solids. So, they do not have fixed shapes like solids. For example, if you take 50ml of water in a cup, it will take the shape of the cup. Now pour the water from the cup to a jug, the liquid has changed its shape and taken the shape of the jug.
The boiling point of a liquid changes with the change in the applied pressure; the normal boiling point of liquid is ${100^ \circ }C$ i.e $({212^ \circ }F)$ . This process of slowly converting liquids into vapour or gaseous phase on heating is called boiling.
Note:
Ensure not to be confused between the terms, intermolecular forces and intramolecular forces. Intramolecular forces, such as covalent and ionic bonds, which are the forces exerted within the molecules to keep the atoms together. Molecules of Liquid are less closely packed as compared to the molecules of solid.
Complete answer:
Liquid is mostly incompressible fluid that upholds the shape of its container. In liquids, molecules are closely packed. Like gas, there molecules do not have lots of space between them. So, they do not disperse to fill every space of a container like gas and have equitably constant density. They are made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds.

Liquids flow because the intermolecular forces between their molecules are weak enough to allow the molecules to move relative to one another. Intermolecular forces are the forces between two adjacent molecules.
Liquids have definite volume but indefinite shape. As the intermolecular attraction between the molecules of liquids are weaker than that of solids. So, they do not have fixed shapes like solids. For example, if you take 50ml of water in a cup, it will take the shape of the cup. Now pour the water from the cup to a jug, the liquid has changed its shape and taken the shape of the jug.
The boiling point of a liquid changes with the change in the applied pressure; the normal boiling point of liquid is ${100^ \circ }C$ i.e $({212^ \circ }F)$ . This process of slowly converting liquids into vapour or gaseous phase on heating is called boiling.
Note:
Ensure not to be confused between the terms, intermolecular forces and intramolecular forces. Intramolecular forces, such as covalent and ionic bonds, which are the forces exerted within the molecules to keep the atoms together. Molecules of Liquid are less closely packed as compared to the molecules of solid.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 7 English: Engaging Questions & Answers for Success

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




