
What are the resonance structures of $S{{O}_{2}}$ ?
Answer
535.5k+ views
Hint: Sulfur dioxide ($S{{O}_{2}}$) has two resonance structures which contribute equally to the overall hybrid structure of the molecule. However, a third Lewis structure can be drawn for $S{{O}_{2}}$ which is more stable in theory rather than being experimentally stable.
Complete answer:
Resonance structure can be defined as any of two or more possible structures for a compound which have identical geometry but different arrangements of the paired electrons. Thus, we can say that the resonating structures are just the way of representing the same molecule in different manners.
-Let us draw the resonance structures of $S{{O}_{2}}$.As we know that sulfur has '4' valence electrons and each oxygen atom has '6' valence electrons. Hence, the total number of valence electrons in $S{{O}_{2}}$ = [4 + 2(6)] = 18
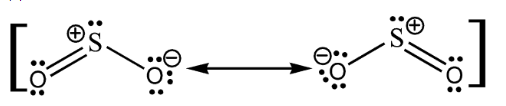
From the above structures we can observe that there are 6 bonding electrons and 12 non-bonding electrons (represented as lone pairs). These 2 resonance structures are equivalent and therefore both of them will contribute equally to the hybrid structure of $S{{O}_{2}}$. These structures have formal charges; the negative formal charge is placed on oxygen atom since it is more electronegative than sulfur, while the positive charge is placed on sulfur. The hybrid structure of $S{{O}_{2}}$ is shown below:-
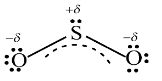
The negative charge will split among two oxygen atoms. Hence, the charges on the atoms are +1.4 for sulfur and -0.7 for each oxygen atom. Also, we can observe that molecule's double bonds have single bond character as well.
Thus, total no. of resonating structures for $S{{O}_{2}}$=2.
Note: The theoretically stable Lewis structure of $S{{O}_{2}}$ is shown below:-
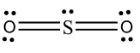
So, theoretically this structure would be more stable than the previous two due to the fact that it has more covalent bonds and no formal charges on any of the atoms. But remember that experimental data would always point towards the hybrid structure.
Complete answer:
Resonance structure can be defined as any of two or more possible structures for a compound which have identical geometry but different arrangements of the paired electrons. Thus, we can say that the resonating structures are just the way of representing the same molecule in different manners.
-Let us draw the resonance structures of $S{{O}_{2}}$.As we know that sulfur has '4' valence electrons and each oxygen atom has '6' valence electrons. Hence, the total number of valence electrons in $S{{O}_{2}}$ = [4 + 2(6)] = 18

From the above structures we can observe that there are 6 bonding electrons and 12 non-bonding electrons (represented as lone pairs). These 2 resonance structures are equivalent and therefore both of them will contribute equally to the hybrid structure of $S{{O}_{2}}$. These structures have formal charges; the negative formal charge is placed on oxygen atom since it is more electronegative than sulfur, while the positive charge is placed on sulfur. The hybrid structure of $S{{O}_{2}}$ is shown below:-

The negative charge will split among two oxygen atoms. Hence, the charges on the atoms are +1.4 for sulfur and -0.7 for each oxygen atom. Also, we can observe that molecule's double bonds have single bond character as well.
Thus, total no. of resonating structures for $S{{O}_{2}}$=2.
Note: The theoretically stable Lewis structure of $S{{O}_{2}}$ is shown below:-
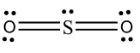
So, theoretically this structure would be more stable than the previous two due to the fact that it has more covalent bonds and no formal charges on any of the atoms. But remember that experimental data would always point towards the hybrid structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




