
Arrange $HCl{O_3}$ , $HBr{O_3}$ , $HI{O_3}$ in order of acidic strength.
A.$HCl{O_3} > HBr{O_3} > HI{O_3}$
B.$HBr{O_3} > HI{O_3} > HCl{O_3}$
C.$HI{O_3} > HBr{O_3} > HCl{O_3}$
D.$HCl{O_3} > HI{O_3} > HBr{O_3}$
Answer
570k+ views
Hint:
Binary acids are molecular compounds in which hydrogen combines with another non-metallic element. These acids include $HCl,HBr,HF,HI$ . $HF$ is a weak acid while all others are strong acids.
Complete step by step answer:
Chlorine, bromine and iodine all belong to the same group(halogen group). In the periodic table as we go down a group, atomic size increases and electronegativity decreases.
Electronegativity is the measure of ability of an atom to pull electrons towards itself.
The strength of an acid is determined from its ability to donate ${H^ + }$ ions. The above given acids have the structure-
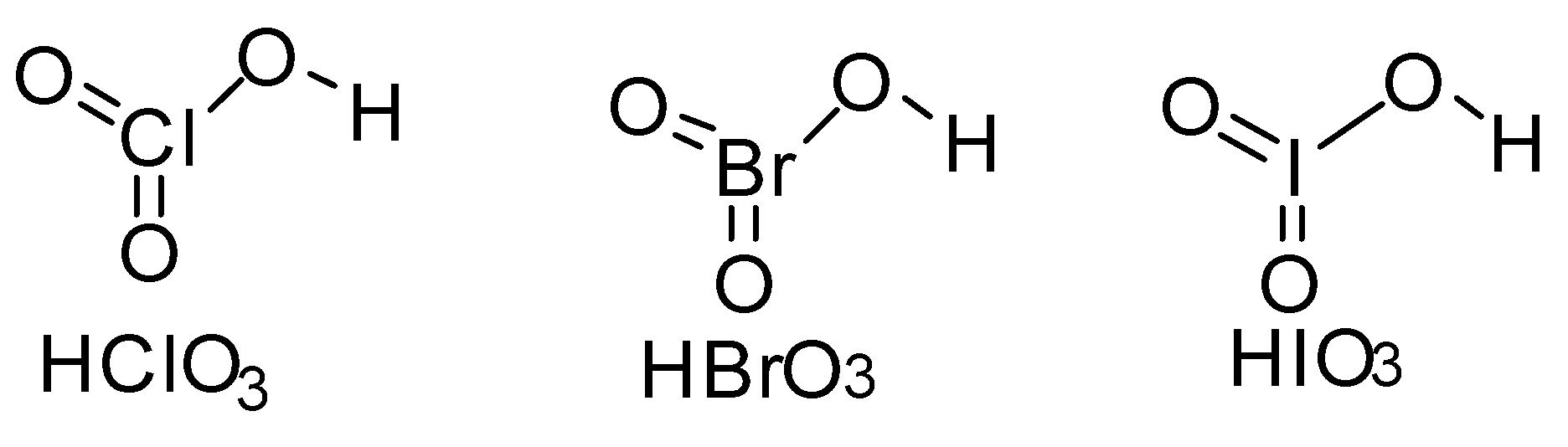
All the above given acids are monobasic acids. They can give one ${H^ + }$ ion in solution. Now in the above acids the weaker the $ - O - H$ bond the more easily ${H^ + }$ ions can ionize in water and stronger will be the acid.
In all the above acids we can see that the hydrogen is attached through oxygen to the electronegative atom like chlorine, bromine and iodine. Electronegativity value plays a major role here. Amongst them chlorine has highest electronegativity. So it will pull the electrons of $ - O - H$ bond more strongly towards itself making hydrogen electron deficient. So ${H^ + }$ ion will be lost easily making $HCl{O_3}$ stronger acid amongst others.
Amongst the three halogen, bromine has electronegativity value less than chlorine and iodine has the least electronegativity value. The hydrogen in $HI{O_3}$ will be least acidic since iodine being the least electronegative among the three will not be able to pull the electrons as strongly as in $HCl{O_3}$ and $HBr{O_3}$ . So $HI{O_3}$ will be least acidic. And $HBr{O_3}$ will have acidic strength more than $HI{O_3}$ but less than $HCl{O_3}$ .
The order of acidic strength will be-
$HCl{O_3} > HBr{O_3} > HI{O_3}$
So the correct option is A.
Note:The strength of an acid varies depending on the solvent in which it is dissolved. Acidic strength is inversely proportional to the basic strength. Acid which is strong in water may be weak in less basic solvent and vice-versa.
Binary acids are molecular compounds in which hydrogen combines with another non-metallic element. These acids include $HCl,HBr,HF,HI$ . $HF$ is a weak acid while all others are strong acids.
Complete step by step answer:
Chlorine, bromine and iodine all belong to the same group(halogen group). In the periodic table as we go down a group, atomic size increases and electronegativity decreases.
Electronegativity is the measure of ability of an atom to pull electrons towards itself.
The strength of an acid is determined from its ability to donate ${H^ + }$ ions. The above given acids have the structure-

All the above given acids are monobasic acids. They can give one ${H^ + }$ ion in solution. Now in the above acids the weaker the $ - O - H$ bond the more easily ${H^ + }$ ions can ionize in water and stronger will be the acid.
In all the above acids we can see that the hydrogen is attached through oxygen to the electronegative atom like chlorine, bromine and iodine. Electronegativity value plays a major role here. Amongst them chlorine has highest electronegativity. So it will pull the electrons of $ - O - H$ bond more strongly towards itself making hydrogen electron deficient. So ${H^ + }$ ion will be lost easily making $HCl{O_3}$ stronger acid amongst others.
Amongst the three halogen, bromine has electronegativity value less than chlorine and iodine has the least electronegativity value. The hydrogen in $HI{O_3}$ will be least acidic since iodine being the least electronegative among the three will not be able to pull the electrons as strongly as in $HCl{O_3}$ and $HBr{O_3}$ . So $HI{O_3}$ will be least acidic. And $HBr{O_3}$ will have acidic strength more than $HI{O_3}$ but less than $HCl{O_3}$ .
The order of acidic strength will be-
$HCl{O_3} > HBr{O_3} > HI{O_3}$
So the correct option is A.
Note:The strength of an acid varies depending on the solvent in which it is dissolved. Acidic strength is inversely proportional to the basic strength. Acid which is strong in water may be weak in less basic solvent and vice-versa.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




