
Arrange the following nitrogen containing compounds in order of basicity?
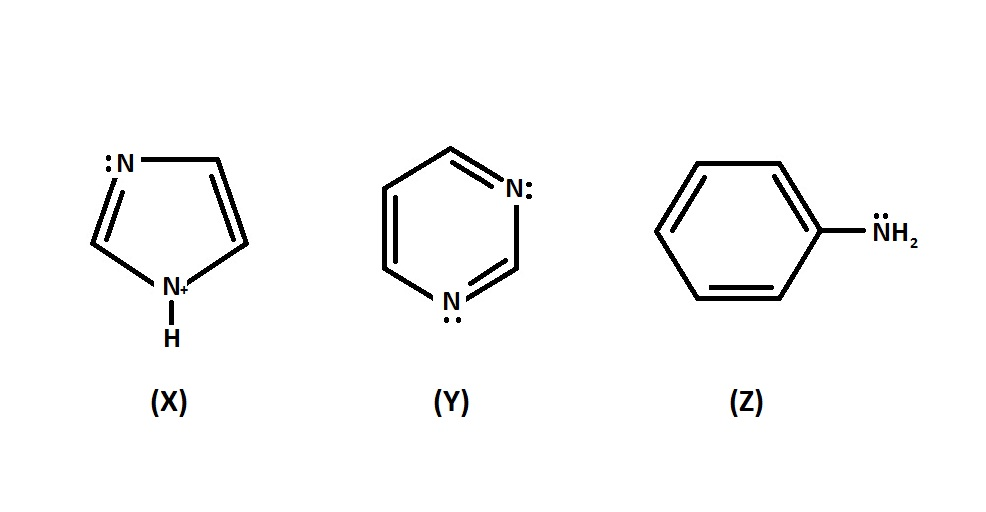
A. X $\, > \,$ Y $\, > \,$Z
B. Y $\, > \,$X $\, > \,$ Z
C. Z $\, > \,$ X $\, > \,$Y
D. X $\, > \,$Z $\, > \,$ Y

Answer
566.7k+ views
Hint: Since amines have a pair of unshared electrons, which they can exchange with other atoms, amines are basic. An electron density around the nitrogen atom is produced by these unshared electrons. The higher the density of electrons, the more basic the molecule is. Basicity of amines increases by electron donating groups and decreased by electron withdrawing groups
Complete step by step answer:
Amines are basic in nature due to the presence of a lone pair of electrons on nitrogen atoms of the $N{H_2}$ group, which it can donate to electron deficient compounds. Aliphatic amines are stronger bases than $N{H_3}$ because of the $ + I$ effect of the alkyl group, greater the number of alkyl groups greater will be the electron density on it and more will be the basicity. Considering the case of aromatic amines shown in the question, we should first know that aromatic amine is organic compound which have aromatic ring attached to amines, aromatic amines are less basic or weaker bases compared to alkyl amines and ammonia because the $\, - N{H_2}\,$ group is attached to a $\, - {C_6}{H_5}\,$ group which is the electron withdrawing group, in aromatic amines. The supply of a lone pair of electrons on $\,N\,$ is thus limited.
Here in this question, every nitrogen has lone pairs . X shows maximum basicity because its aliphatic amine (as this do not contain aromatic character, since it does not obey Huckel’s rule) and Z is least basic because $N$ on the compound Aniline is participated in delocalization over the aromatic ring and it is less readily available for protonation as compared to Y.
Therefore the order of basicity is X $\, > \,$ Y $\, > \,$Z
So, the correct answer is Option A.
Note: The basicity of heterocyclic amines depends on the position of the nitrogen atom electron pair, its hybridization, and whether or not it is possible to stabilise the resonance. Basicity is expressed by means of the $\,{K_b}\,$ values determined by the amine reaction with water. An alternative basicity measure is $\,p{K_b}\,$, which is $\, - \log {K_b}\,$. A big $\,{K_b}\,$ and a small $\,p{K_b}\,$shows a strong base. The acidity of their conjugate acids also reflects the basicity of amines. A strong base, shows small$\,{K_a}\,$ value and a large $\,p{K_a}\,$ by its weak conjugate acid.
Complete step by step answer:
Amines are basic in nature due to the presence of a lone pair of electrons on nitrogen atoms of the $N{H_2}$ group, which it can donate to electron deficient compounds. Aliphatic amines are stronger bases than $N{H_3}$ because of the $ + I$ effect of the alkyl group, greater the number of alkyl groups greater will be the electron density on it and more will be the basicity. Considering the case of aromatic amines shown in the question, we should first know that aromatic amine is organic compound which have aromatic ring attached to amines, aromatic amines are less basic or weaker bases compared to alkyl amines and ammonia because the $\, - N{H_2}\,$ group is attached to a $\, - {C_6}{H_5}\,$ group which is the electron withdrawing group, in aromatic amines. The supply of a lone pair of electrons on $\,N\,$ is thus limited.
Here in this question, every nitrogen has lone pairs . X shows maximum basicity because its aliphatic amine (as this do not contain aromatic character, since it does not obey Huckel’s rule) and Z is least basic because $N$ on the compound Aniline is participated in delocalization over the aromatic ring and it is less readily available for protonation as compared to Y.
Therefore the order of basicity is X $\, > \,$ Y $\, > \,$Z
So, the correct answer is Option A.
Note: The basicity of heterocyclic amines depends on the position of the nitrogen atom electron pair, its hybridization, and whether or not it is possible to stabilise the resonance. Basicity is expressed by means of the $\,{K_b}\,$ values determined by the amine reaction with water. An alternative basicity measure is $\,p{K_b}\,$, which is $\, - \log {K_b}\,$. A big $\,{K_b}\,$ and a small $\,p{K_b}\,$shows a strong base. The acidity of their conjugate acids also reflects the basicity of amines. A strong base, shows small$\,{K_a}\,$ value and a large $\,p{K_a}\,$ by its weak conjugate acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




