
Assertion: A solution of sucrose in water is dextrorotatory but on hydrolysis in the presence of \[\text{ }{{\text{H}}^{\oplus }}\text{ }\] , it becomes laevorotatory.
Reason: Inversion of sugar follows first-order kinetics.
(A) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
(B) Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
(C) Assertion is correct but Reason is incorrect
(D) Both Assertion and Reason are incorrect
Answer
584.7k+ views
Hint: The sucrose is a disaccharide of glucose and fructose. On hydrolysis, the glycosidic linkage ruptures to generate the optical isomers of glucose and fructose. The kinetics of the reaction depends on the concentration of the reactant or product.
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow{\text{HCl}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Sucrose)} & {} & {} & {} & \text{(D-Glucose)} & {} & \text{(D-Fructose)} \\
\end{matrix}$
The inversion of sugar does not depend on the concentrations of sugar.
Complete step by step solution:
Sucrose is a common sugar. Sucrose is a disaccharide composed of two monosaccharides glucose and fructose. It is a $\text{ 1:1 }$ mixture of glucose and fructose. The glucose and fructose are joined together by the glycosidic bond.
The glucose and fructose exist in the $\text{ }\alpha \text{ }$ and $\text{ }\beta \text{ }$ form. However, to form a glycosidic linkage to form sucrose the $\text{ }\alpha \text{ }$form of the glucose links to the $\text{ }\beta \text{ }$form of fructose. The purity of sucrose is measured by the polarimetry. It rotates the plane-polarized light in the clockwise direction. It has a specific rotation of $\text{ }+66.47{}^\circ \text{ }$ . Before hydrolysis the sucrose is dextrorotatory. The glucose and fructose have optical isomers.
Assume that sucrose is treated with a mineral acid such as$\text{ HCl }$. The acid provides the proton which facilitates the hydrolysis of sucrose.
The glucose and fructose are linked by the acetal oxygen bridge $\text{ }-\text{O}-\text{ }$ . This bride links the carbon at 1 position of the $\text{ }\alpha \text{ }$glucose to the carbon of the 2 positions of the $\text{ }\beta \text{ }$ fructose.
During the hydrolysis reaction, the glycosidic linkage between the glucose and fructose breaks down. The bond is broken in such a way that the H from the acid (or water) is added to the oxygen of the glucose. The $-\text{OH}$ group is added to the carbon of the fructose.
The reaction is as shown below,
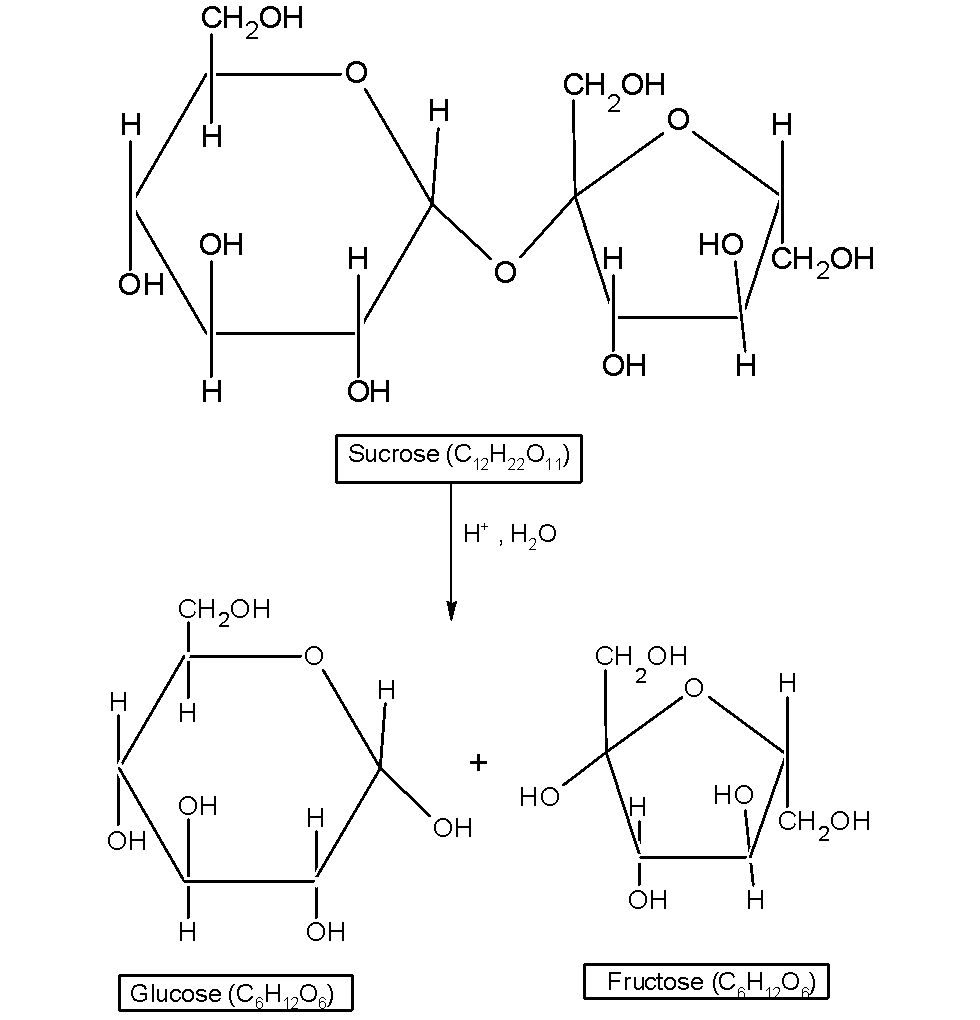
On hydrolysis, the sucrose breaks down to give the dextrorotatory glucose and laevorotatory fructose. The dextrorotation of the glucose is $\text{ }+52.5{}^\circ \text{ }$and the laevorotation of the fructose is by$\text{ -90}\text{.4}{}^\circ \text{ }$. That is the optical isomer of the glucose and fructose changes after the hydrolysis reaction.
Since sucrose is made of glucose and fructose, the change in the specific rotation of the monosaccharides results in the change in the specific rotation of sucrose. On hydrolysis, the sucrose solution is laevorotatory.
Therefore, a solution of sucrose in water is dextrorotatory but on hydrolysis in the presence of an acid, it becomes laevorotatory.
So, the assertion is correct.
The sucrose breaks down into the glucose and fructose in presence of an acidic medium. the general reaction is depicted as follows,
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow{\text{HCl}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Sucrose)} & {} & {} & {} & \text{(D-Glucose)} & {} & \text{(D-Fructose)} \\
\end{matrix}$
The rate of formation of glucose and fructose or the rate of the reaction is directly proportional to the concentration of sucrose and acid.
The rate can be expressed as,
\[\text{ Rate of reaction }\propto \text{ }\left[ {{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} \right]\left[ {{\text{H}}_{\text{2}}}\text{O} \right]\text{ }\]
The reaction may look like a second order as it depends on the concentration of two species. However the reaction is carried out in the water which also acts as the solvent. It is present in the larger quantity. Thus, the water is taken as the constant. Now, the rate of reaction of the inversion of sugar depends on the concentration of sucrose only.
\[\text{ Rate of reaction =K }\left[ {{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} \right]\]
Therefore, the reaction follows first-order kinetic.
So, the reason is also a correct statement.
However, the reason is not a proper explanation for the assertion.
Hence, (B) is the correct option.
Note: Note that, on hydrolysis, the laevorotation (or fructose) is more than the dextrorotation (of glucose) thus the solution has the resultant rotation as the leavo rotation. On hydrolysis, the specific rotation of sucrose brings a change in sign thus the sucrose is inverted sugar. The reaction of hydrolysis of sucrose is more precisely a pseudo-first-order reaction.
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow{\text{HCl}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Sucrose)} & {} & {} & {} & \text{(D-Glucose)} & {} & \text{(D-Fructose)} \\
\end{matrix}$
The inversion of sugar does not depend on the concentrations of sugar.
Complete step by step solution:
Sucrose is a common sugar. Sucrose is a disaccharide composed of two monosaccharides glucose and fructose. It is a $\text{ 1:1 }$ mixture of glucose and fructose. The glucose and fructose are joined together by the glycosidic bond.
The glucose and fructose exist in the $\text{ }\alpha \text{ }$ and $\text{ }\beta \text{ }$ form. However, to form a glycosidic linkage to form sucrose the $\text{ }\alpha \text{ }$form of the glucose links to the $\text{ }\beta \text{ }$form of fructose. The purity of sucrose is measured by the polarimetry. It rotates the plane-polarized light in the clockwise direction. It has a specific rotation of $\text{ }+66.47{}^\circ \text{ }$ . Before hydrolysis the sucrose is dextrorotatory. The glucose and fructose have optical isomers.
Assume that sucrose is treated with a mineral acid such as$\text{ HCl }$. The acid provides the proton which facilitates the hydrolysis of sucrose.
The glucose and fructose are linked by the acetal oxygen bridge $\text{ }-\text{O}-\text{ }$ . This bride links the carbon at 1 position of the $\text{ }\alpha \text{ }$glucose to the carbon of the 2 positions of the $\text{ }\beta \text{ }$ fructose.
During the hydrolysis reaction, the glycosidic linkage between the glucose and fructose breaks down. The bond is broken in such a way that the H from the acid (or water) is added to the oxygen of the glucose. The $-\text{OH}$ group is added to the carbon of the fructose.
The reaction is as shown below,

On hydrolysis, the sucrose breaks down to give the dextrorotatory glucose and laevorotatory fructose. The dextrorotation of the glucose is $\text{ }+52.5{}^\circ \text{ }$and the laevorotation of the fructose is by$\text{ -90}\text{.4}{}^\circ \text{ }$. That is the optical isomer of the glucose and fructose changes after the hydrolysis reaction.
Since sucrose is made of glucose and fructose, the change in the specific rotation of the monosaccharides results in the change in the specific rotation of sucrose. On hydrolysis, the sucrose solution is laevorotatory.
Therefore, a solution of sucrose in water is dextrorotatory but on hydrolysis in the presence of an acid, it becomes laevorotatory.
So, the assertion is correct.
The sucrose breaks down into the glucose and fructose in presence of an acidic medium. the general reaction is depicted as follows,
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow{\text{HCl}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Sucrose)} & {} & {} & {} & \text{(D-Glucose)} & {} & \text{(D-Fructose)} \\
\end{matrix}$
The rate of formation of glucose and fructose or the rate of the reaction is directly proportional to the concentration of sucrose and acid.
The rate can be expressed as,
\[\text{ Rate of reaction }\propto \text{ }\left[ {{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} \right]\left[ {{\text{H}}_{\text{2}}}\text{O} \right]\text{ }\]
The reaction may look like a second order as it depends on the concentration of two species. However the reaction is carried out in the water which also acts as the solvent. It is present in the larger quantity. Thus, the water is taken as the constant. Now, the rate of reaction of the inversion of sugar depends on the concentration of sucrose only.
\[\text{ Rate of reaction =K }\left[ {{\text{C}}_{\text{12}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{11}}} \right]\]
Therefore, the reaction follows first-order kinetic.
So, the reason is also a correct statement.
However, the reason is not a proper explanation for the assertion.
Hence, (B) is the correct option.
Note: Note that, on hydrolysis, the laevorotation (or fructose) is more than the dextrorotation (of glucose) thus the solution has the resultant rotation as the leavo rotation. On hydrolysis, the specific rotation of sucrose brings a change in sign thus the sucrose is inverted sugar. The reaction of hydrolysis of sucrose is more precisely a pseudo-first-order reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




