
Assertion: Both o-hydroxybenzaldehyde and p-hydroxy benzaldehyde have the same molecular weight and show H-bonding.
Reason: Melting point of p-hydroxybenzaldehyde is more than o-hydroxybenzaldehyde.
A. Both assertion and reason are correct and reason is the correct explanation of the assertion.
B. Both assertion and reason are correct but reason is not the correct explanation of the assertion.
C. Assertion is correct but reason is incorrect.
D. Both assertion and reason are incorrect.
Answer
544.2k+ views
Hint:In the above question, some assertion o-hydroxybenzaldehyde and p-hydroxybenzaldehyde about is given. We are provided with the information about their melting point. We have to check which of the following options suits the best. The basic difference between o-hydroxybenzaldehyde and p-hydroxybenzaldehyde lies in the position of ${\text{ - OH}}$ group with respect to ${\text{ - CHO}}$ group which makes them differ in chemical characteristics as well.
Complete step-by-step answer:Both ortho-hydroxybenzaldehyde (o-hydroxybenzaldehyde) and para-hydroxy benzaldehyde (p-hydroxybenzaldehyde) are the isomers of hydroxyl benzaldehyde. And hence, the molecular weight of the two compounds are the same and both of them show H-bonding. So, the assertion is correct.
In o-hydroxybenzaldehyde, if the ${\text{ - CHO}}$ group is present at 1 location of the benzene ring then ${\text{ - OH}}$ group is present at 2 locations. Due to the presence of H-atoms closer to each other, they show intramolecular hydrogen bonding.
In p-hydroxybenzaldehyde, if the ${\text{ - CHO}}$ group is present at 1 location of the benzene ring then ${\text{ - OH}}$ group is present at 4 locations. Due to absence of H-atoms closer to each other, they show intermolecular hydrogen bonding.
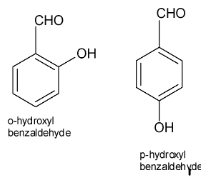
Due to intermolecular hydrogen bonding or the bonding between adjacent molecules, the melting point of p-hydroxybenzaldehyde is more than o-benzaldehyde.
Since, both assertion and reason are correct but reason is not the correct explanation of the assertion.
b>Therefore, the correct option is option B.
Note:Although o-hydroxybenzaldehyde and p-hydroxy benzaldehyde have the same molecular mass and chemical formula, still they differ significantly due to difference in the type of their H-bonding. To answer any question related to ortho and para type question, answer with respect to intermolecular and intermolecular H-bonding.
Complete step-by-step answer:Both ortho-hydroxybenzaldehyde (o-hydroxybenzaldehyde) and para-hydroxy benzaldehyde (p-hydroxybenzaldehyde) are the isomers of hydroxyl benzaldehyde. And hence, the molecular weight of the two compounds are the same and both of them show H-bonding. So, the assertion is correct.
In o-hydroxybenzaldehyde, if the ${\text{ - CHO}}$ group is present at 1 location of the benzene ring then ${\text{ - OH}}$ group is present at 2 locations. Due to the presence of H-atoms closer to each other, they show intramolecular hydrogen bonding.
In p-hydroxybenzaldehyde, if the ${\text{ - CHO}}$ group is present at 1 location of the benzene ring then ${\text{ - OH}}$ group is present at 4 locations. Due to absence of H-atoms closer to each other, they show intermolecular hydrogen bonding.

Due to intermolecular hydrogen bonding or the bonding between adjacent molecules, the melting point of p-hydroxybenzaldehyde is more than o-benzaldehyde.
Since, both assertion and reason are correct but reason is not the correct explanation of the assertion.
b>Therefore, the correct option is option B.
Note:Although o-hydroxybenzaldehyde and p-hydroxy benzaldehyde have the same molecular mass and chemical formula, still they differ significantly due to difference in the type of their H-bonding. To answer any question related to ortho and para type question, answer with respect to intermolecular and intermolecular H-bonding.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




