
Assertion: Cyclopentadienyl anion is aromatic in nature.
Reason: Cyclopentadienyl has six $\pi $ electrons.
A.Both assertion and reason are correct and Reason is the correct explanation for Assertion.
B.Both assertion and reason are correct and Reason is the correct explanation for Assertion.
C.Assertion is correct but Reason is incorrect.
D.Both Assertion and Reason are incorrect.
Answer
569.1k+ views
Hint:
In this question, we have to discuss the aromatic nature of compounds and according to which they select the option for correctness for both assertion and reason or not.
Complete step by step answer:
-Aromatic Compounds: Aromatic compounds are those chemical compounds which contain conjugated planar ring systems along with delocalized pi electron clouds. For example, benzene and toluene are aromatic compounds.
-For determining the Aromatic nature of compounds, the Huckel Rule is used. Huckel proposed a theory to help determine if a planar ring molecule would have aromatic properties or not.
-This rule states that if a cyclic, planar molecule has $\left( {4n\, + \,2\,\pi } \right)$electrons, it is considered AROMATIC.
Four Conditions for AROMATICITY
1.The molecule is cyclic (a ring of atoms)
2.The molecule is planar (all atoms in the molecule lie in the same plane)
3.The molecule is fully conjugated (p orbitals at every atom in the ring)
4.The molecule has $\left( {4n\, + \,2\,\pi } \right)$electrons ($n = 0$or any positive integer)
So, According to the Question,
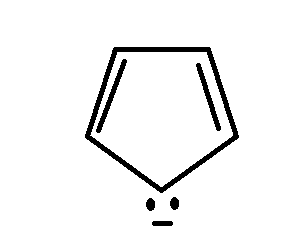
For the given compound if we put $n = 1$ in Huckel's rule, we get
$\left( {4\, \times \,1\, + \,2\pi } \right)\, = 6\pi $
Hence it satisfies Huckel's rule
Also, cyclopentadienyl anion is planar in nature and $(4n + 2\pi )$ electrons that are delocalised over the entire ring.
Hence, cyclopentadienyl anion is aromatic in nature and hence the assertion is correct.
Also, it contains six $\pi $ electrons because of which it is aromatic.
Therefore, Both assertion and reason are correct and Reason is the correct explanation for Assertion.
Hence, option A is correct.
Note: For the aromatic nature of compound, Huckel rule should be followed by applying the above four conditions, without these conditions no compound can be treated as aromatic. These molecules are highly stable and do not break apart easily to react with other substances.
In this question, we have to discuss the aromatic nature of compounds and according to which they select the option for correctness for both assertion and reason or not.
Complete step by step answer:
-Aromatic Compounds: Aromatic compounds are those chemical compounds which contain conjugated planar ring systems along with delocalized pi electron clouds. For example, benzene and toluene are aromatic compounds.
-For determining the Aromatic nature of compounds, the Huckel Rule is used. Huckel proposed a theory to help determine if a planar ring molecule would have aromatic properties or not.
-This rule states that if a cyclic, planar molecule has $\left( {4n\, + \,2\,\pi } \right)$electrons, it is considered AROMATIC.
Four Conditions for AROMATICITY
1.The molecule is cyclic (a ring of atoms)
2.The molecule is planar (all atoms in the molecule lie in the same plane)
3.The molecule is fully conjugated (p orbitals at every atom in the ring)
4.The molecule has $\left( {4n\, + \,2\,\pi } \right)$electrons ($n = 0$or any positive integer)
So, According to the Question,

For the given compound if we put $n = 1$ in Huckel's rule, we get
$\left( {4\, \times \,1\, + \,2\pi } \right)\, = 6\pi $
Hence it satisfies Huckel's rule
Also, cyclopentadienyl anion is planar in nature and $(4n + 2\pi )$ electrons that are delocalised over the entire ring.
Hence, cyclopentadienyl anion is aromatic in nature and hence the assertion is correct.
Also, it contains six $\pi $ electrons because of which it is aromatic.
Therefore, Both assertion and reason are correct and Reason is the correct explanation for Assertion.
Hence, option A is correct.
Note: For the aromatic nature of compound, Huckel rule should be followed by applying the above four conditions, without these conditions no compound can be treated as aromatic. These molecules are highly stable and do not break apart easily to react with other substances.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




