
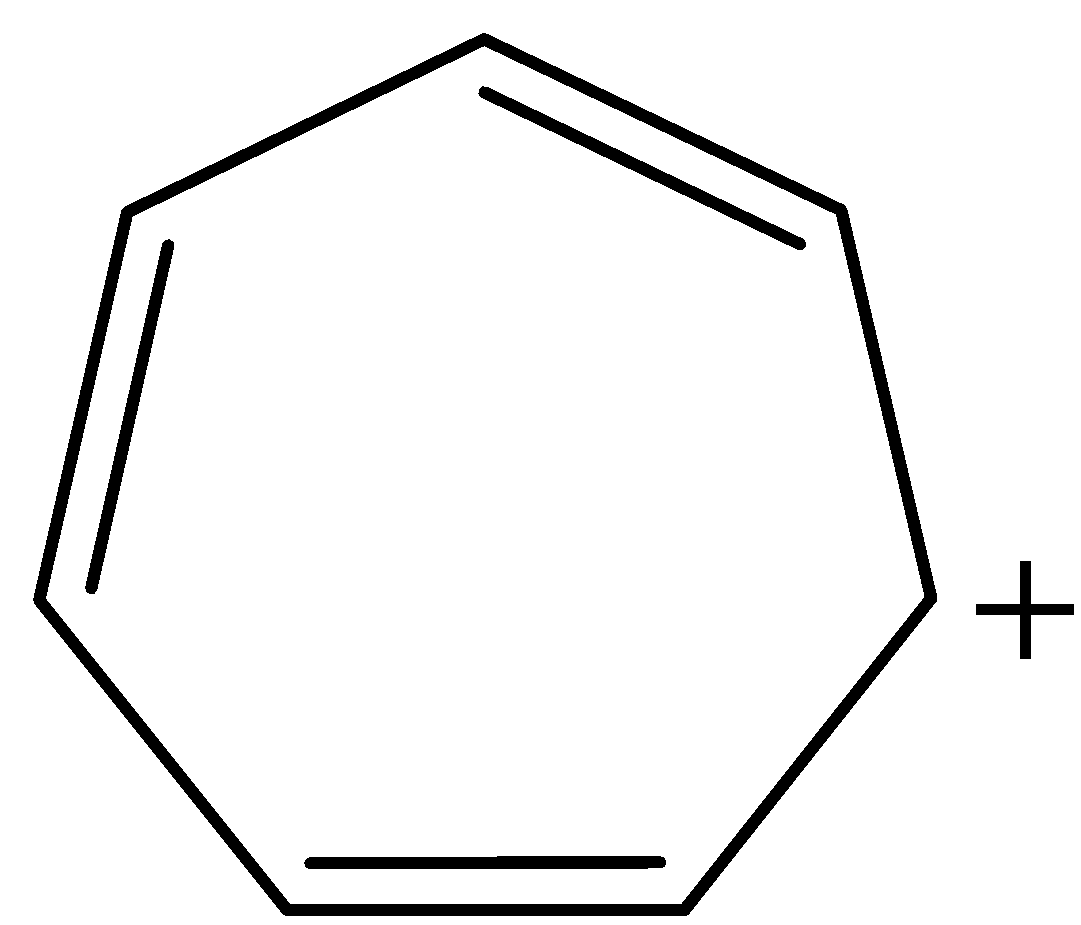
Assertion – Tropylium cation is aromatic in nature (refer image)
Reason: The only property that determines its aromatic behaviour is its planar structure.
A) Both assertion and reason are correct and reason is the correct explanation for assertion.
B) Both assertion and reason are correct but reason is not the correct explanation for assertion.
C) Assertion is correct but reason is incorrect.
D) Assertion is incorrect and reason is correct.

Answer
585.6k+ views
Hint: Recall the necessary conditions required for any system to be aromatic. According to Huckel’s rule, if a cyclic, planar molecule has $(4n + 2)\pi $ electrons in conjugation, it is considered aromatic.
Complete step by step answer:
In 1931, German chemist and physicist Erich Huckel proposed his theory to help determine if a ring is aromatic or not. His rule known as Huckel’s rule states that when the number of $\pi $ electrons equals $(4n + 2)\pi $, where n is a non-negative integer, in a cyclic, planar system, it is considered as an aromatic system.
Four necessary conditions required for aromaticity:
- The molecule must be cyclic that is, a ring of atoms.
- The molecule must be planar. It means all atoms in the molecule must lie in the same plane.
- All the atoms must be $s{p^2}$ hybridised and all the p-orbitals in the ring must be perpendicular to each other.
- The cyclic system must have $(4n + 2)\pi $ electrons and they are in continuous delocalisation.
Now, coming to our given structure of tropylium cation:
The given structure is cyclic and planar. All the atoms in the ring are $s{p^2}$ hybridised and all the pi-electrons are in conjugation with positive charge, that is, there is complete delocalisation of pi-electrons. Number of $\pi $ electrons in the ring are 6, therefore, tropylium cation also follows Huckel's Rule of $(4n + 2)\pi $ electrons, where n = 1. Thus, tropylium cation is satisfying all the conditions for aromaticity and consequently, aromatic in nature.
Hence summarising the above explanation, both the given assertion and reason are correct and reason is the correct explanation of assertion.
Thus, option A is correct.
Note: Tropylium cation also known with a common name as cycloheptatrienyl cation. Its IUPAC name is 2,4,6-cycloheptatrienyl ium. The structure of tropylium cation is a combination of seven resonance contributors in which each carbon atom carries part of the positive charge.
Complete step by step answer:
In 1931, German chemist and physicist Erich Huckel proposed his theory to help determine if a ring is aromatic or not. His rule known as Huckel’s rule states that when the number of $\pi $ electrons equals $(4n + 2)\pi $, where n is a non-negative integer, in a cyclic, planar system, it is considered as an aromatic system.
Four necessary conditions required for aromaticity:
- The molecule must be cyclic that is, a ring of atoms.
- The molecule must be planar. It means all atoms in the molecule must lie in the same plane.
- All the atoms must be $s{p^2}$ hybridised and all the p-orbitals in the ring must be perpendicular to each other.
- The cyclic system must have $(4n + 2)\pi $ electrons and they are in continuous delocalisation.
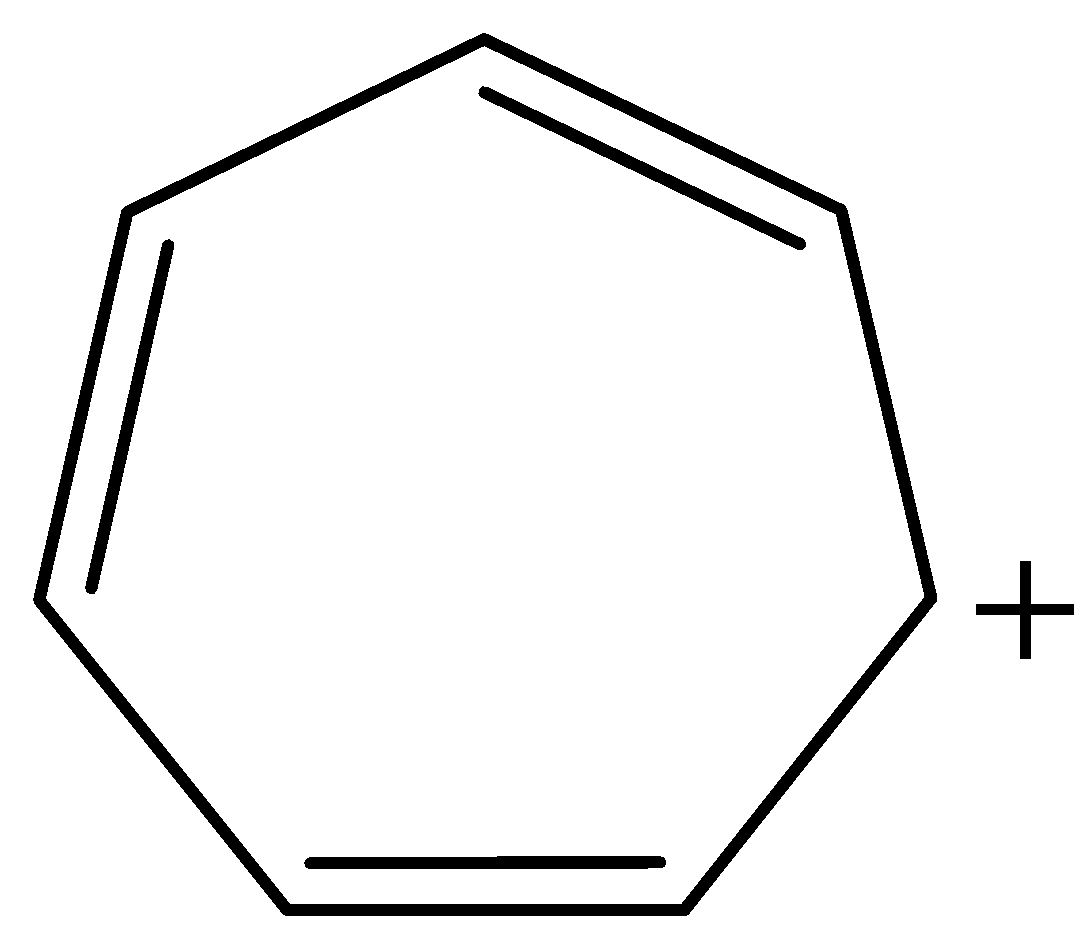
Now, coming to our given structure of tropylium cation:

The given structure is cyclic and planar. All the atoms in the ring are $s{p^2}$ hybridised and all the pi-electrons are in conjugation with positive charge, that is, there is complete delocalisation of pi-electrons. Number of $\pi $ electrons in the ring are 6, therefore, tropylium cation also follows Huckel's Rule of $(4n + 2)\pi $ electrons, where n = 1. Thus, tropylium cation is satisfying all the conditions for aromaticity and consequently, aromatic in nature.
Hence summarising the above explanation, both the given assertion and reason are correct and reason is the correct explanation of assertion.
Thus, option A is correct.
Note: Tropylium cation also known with a common name as cycloheptatrienyl cation. Its IUPAC name is 2,4,6-cycloheptatrienyl ium. The structure of tropylium cation is a combination of seven resonance contributors in which each carbon atom carries part of the positive charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




