
Atomic size of II A elements from Be to Ra:
A.Increases
B.Decreases
C.Remain constant
D.None of these
Answer
578.7k+ views
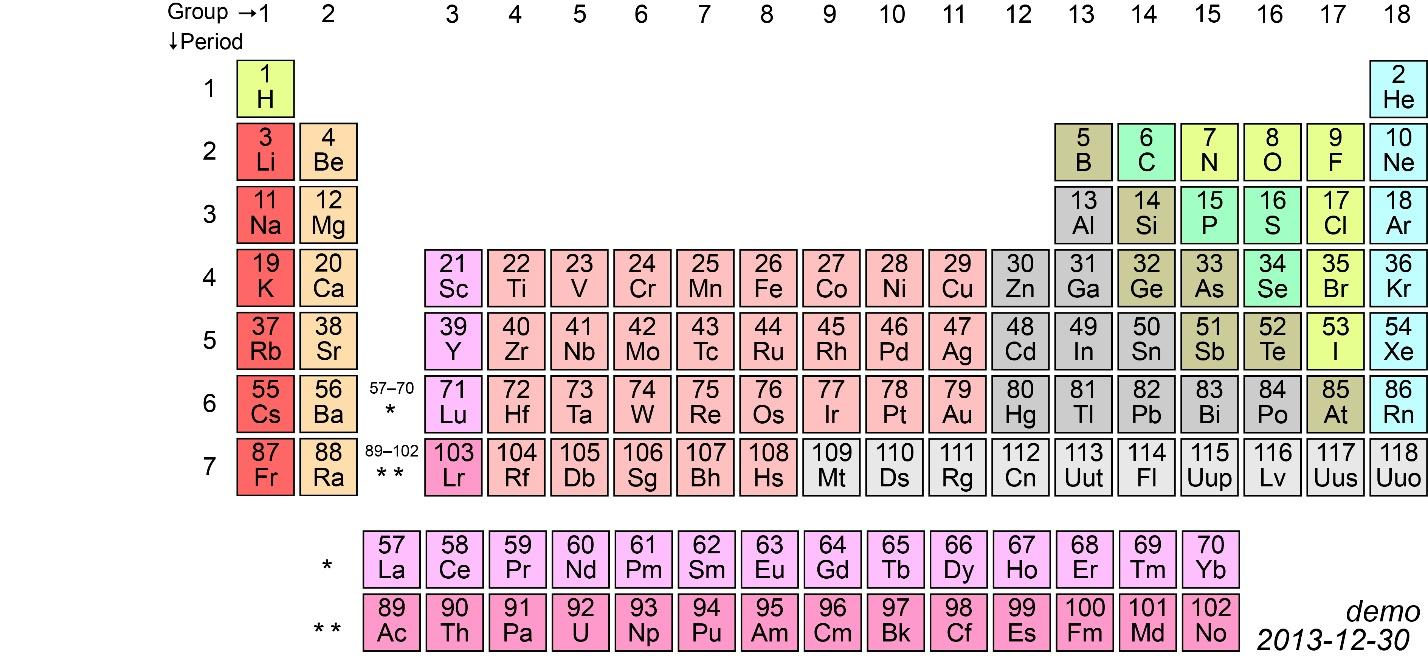
Hint: To solve this question you need to know the trends of a periodic table . You also need to know the position of the elements ( in this case it is already mentioned ) to determine their atomic radius, electronegativity, metallic character, non-metallic character and so on. In this question you have to determine the trend of atomic radius. But first look at the periodic table below, see the elements and guess the correct answer.
Complete step by step answer:
Now, before proceeding any further, you must know what atomic radius is. Atomic size is the distance between the nucleus and the last orbit also known as valence shell. Now from the periodic table you must have by now understood the positions of the elements. Now, let me explain. I hope you can see the number mentioned above every element name. Like for example it is \[4\] for \[Be\] then \[12\] for \[Mg\] and so on. These numbers are the atomic number of the elements. In case you do not know what an atomic number is, an atomic number is defined as the number of protons present in the nucleus of an atom. Now, in a neutral atom, the number of protons is always equal to the number of electrons. So, the atomic number helps us to determine the electronic configuration of the elements which further helps us in finding out the number of orbits. The number of electrons in each orbit is in the format of \[2{n^2}\].For example: electronic configuration of \[Be\] is 2,2 [ putting \[n = 1\] in \[2{n^2}\]we get \[2\] electrons in the first orbit and the capacity of second orbit is \[8\] putting \[n = 2\] in \[2{n^2}\], but since the atomic number is \[4\],the last orbit will have the remaining the number of electrons.] So, the number of orbits is equal is \[2\].Similarly, we find out the the number of orbits of \[Mg\], \[Ca\],\[Sr\] and so on which is 3, 4 ,5 and so one. So, more the atomic number, more is the number of electrons and more is the number of orbits. More is the number of orbits then farther is the last orbit from the nucleus. So greater is the atomic size . We can also notice from the periodic table that atomic number increases down the group. Since atomic size is directly proportional to atomic number. So atomic size also increases down the group from \[Be\] to \[Ra\] .
Thus the correct answer is Option (A).
Note:
Apart from the trends mentioned in this question, we also have other periodic table trends and they are also very important. Like, electron affinity, electronegativity and ionization potential increases across a period from left to right. Metallic character increases down the group. Non-metallic character increases across a period from left to right.

Complete step by step answer:
Now, before proceeding any further, you must know what atomic radius is. Atomic size is the distance between the nucleus and the last orbit also known as valence shell. Now from the periodic table you must have by now understood the positions of the elements. Now, let me explain. I hope you can see the number mentioned above every element name. Like for example it is \[4\] for \[Be\] then \[12\] for \[Mg\] and so on. These numbers are the atomic number of the elements. In case you do not know what an atomic number is, an atomic number is defined as the number of protons present in the nucleus of an atom. Now, in a neutral atom, the number of protons is always equal to the number of electrons. So, the atomic number helps us to determine the electronic configuration of the elements which further helps us in finding out the number of orbits. The number of electrons in each orbit is in the format of \[2{n^2}\].For example: electronic configuration of \[Be\] is 2,2 [ putting \[n = 1\] in \[2{n^2}\]we get \[2\] electrons in the first orbit and the capacity of second orbit is \[8\] putting \[n = 2\] in \[2{n^2}\], but since the atomic number is \[4\],the last orbit will have the remaining the number of electrons.] So, the number of orbits is equal is \[2\].Similarly, we find out the the number of orbits of \[Mg\], \[Ca\],\[Sr\] and so on which is 3, 4 ,5 and so one. So, more the atomic number, more is the number of electrons and more is the number of orbits. More is the number of orbits then farther is the last orbit from the nucleus. So greater is the atomic size . We can also notice from the periodic table that atomic number increases down the group. Since atomic size is directly proportional to atomic number. So atomic size also increases down the group from \[Be\] to \[Ra\] .
Thus the correct answer is Option (A).
Note:
Apart from the trends mentioned in this question, we also have other periodic table trends and they are also very important. Like, electron affinity, electronegativity and ionization potential increases across a period from left to right. Metallic character increases down the group. Non-metallic character increases across a period from left to right.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




