
Benzene cannot undergo-
(A)Substitution
(B)Addition
(C)Elimination
(D)Oxidation
Answer
500.4k+ views
Hint: Benzene is an Aromatic Compound which is colorless or yellow volatile and flammable liquid at room temperature. Its formula is ${C_6}{H_6}$. Its structure consists of 6 membered rings with alternate single and double bonds. Benzene dissolves only slightly in water and Infact floats on its surface.
Complete answer:
Benzene is highly stable as compared to Aliphatic Unsaturated hydrocarbons. The extra stability of Benzene is due to the resonance or the delocalization of electrons across the complete ring. The structure of benzene as proposed by Kekule looks like-
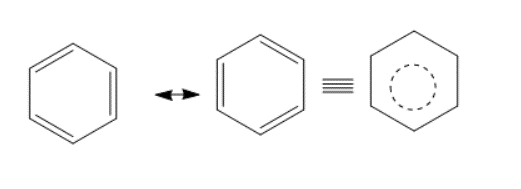
However, none of these structures are exact or accurate. These are the 2 resonating interconverting structures and the last structure is the hybrid structure. Benzene is stable by 36 kcal/mole.
Due to this high stability of Benzene it is highly unreactive as compared to Alkenes and mostly shows reactions under extreme situations only.
In the question we have been asked to name a reaction type benzene does not undergo, so let us closely look at the options-
Option (A): Substitution- Benzene can undergo substitution reaction. In this reaction Hydrogen of Benzene is replaced by Electrophile. Example-Halogenation, Sulphonation, Nitration, Friedel craft’s reaction etc. So this option is eliminated. These are characteristic reactions of benzene.
Option (B): Addition- Benzene generally resists addition reaction because that would cause the loss of stability of benzene however still there are several reactions in which addition occurs. Example- addition of hydrogen and Nickel/Palladium catalyst to benzene producing cyclohexane, preparation of benzene hexachloride from benzene by the reaction with Chlorine and sunlight etc. So this option is also eliminated.
Option(C): Elimination- There is no such evidence of elimination reaction in Benzene since it completely destroys the stability of Benzene.
Option (D): Oxidation- Benzene can undergo the process of Oxidation and produce Phenols, Catechol, maleic anhydride etc. depending on reaction conditions. So this option is also eliminated.
So, option(C) is correct.
Note:
Benzene is a hydrocarbon that is either obtained naturally from forest fires and volcanoes or synthetically produced in the laboratories. This compound is of high industrial significance. It is useful to produce dyes, detergents, resins, plastics, drugs etc. Also benzene forms a major part of gasoline.
Complete answer:
Benzene is highly stable as compared to Aliphatic Unsaturated hydrocarbons. The extra stability of Benzene is due to the resonance or the delocalization of electrons across the complete ring. The structure of benzene as proposed by Kekule looks like-

However, none of these structures are exact or accurate. These are the 2 resonating interconverting structures and the last structure is the hybrid structure. Benzene is stable by 36 kcal/mole.
Due to this high stability of Benzene it is highly unreactive as compared to Alkenes and mostly shows reactions under extreme situations only.
In the question we have been asked to name a reaction type benzene does not undergo, so let us closely look at the options-
Option (A): Substitution- Benzene can undergo substitution reaction. In this reaction Hydrogen of Benzene is replaced by Electrophile. Example-Halogenation, Sulphonation, Nitration, Friedel craft’s reaction etc. So this option is eliminated. These are characteristic reactions of benzene.
Option (B): Addition- Benzene generally resists addition reaction because that would cause the loss of stability of benzene however still there are several reactions in which addition occurs. Example- addition of hydrogen and Nickel/Palladium catalyst to benzene producing cyclohexane, preparation of benzene hexachloride from benzene by the reaction with Chlorine and sunlight etc. So this option is also eliminated.
Option(C): Elimination- There is no such evidence of elimination reaction in Benzene since it completely destroys the stability of Benzene.
Option (D): Oxidation- Benzene can undergo the process of Oxidation and produce Phenols, Catechol, maleic anhydride etc. depending on reaction conditions. So this option is also eliminated.
So, option(C) is correct.
Note:
Benzene is a hydrocarbon that is either obtained naturally from forest fires and volcanoes or synthetically produced in the laboratories. This compound is of high industrial significance. It is useful to produce dyes, detergents, resins, plastics, drugs etc. Also benzene forms a major part of gasoline.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




