
Benzyl amine reacts with nitrous acid to form mainly?
A. Phenol
B. Benzyl alcohol
C. Benzaldehyde
D. Nitrobenzene
Answer
571.8k+ views
Hint: Nitrous acid is an oxidizing agent. It oxidizes the amine group into diazonium salt. The diazonium salts release nitrogen gas and form electrophile. In. In the presence of water the electrophile is attacked by hydroxide ions.
Complete step by step solution: Benzyl amine is also known as aniline. The reaction of benzyl amine with nitrous acid gives benzene diazonium salt. The amine group has a lone pair of electrons, so it attacks on nitrous acid. By this reaction, dinitrogen adds to the benzene ring. The formed salt is known as diazonium salt.
The dinitrogen has a positive charge. Release and form benzene cation which is an electrophile. In this electrophilic ring, the hydroxyl group (nucleophile) attacks to give phenol.
The reaction is as follows:
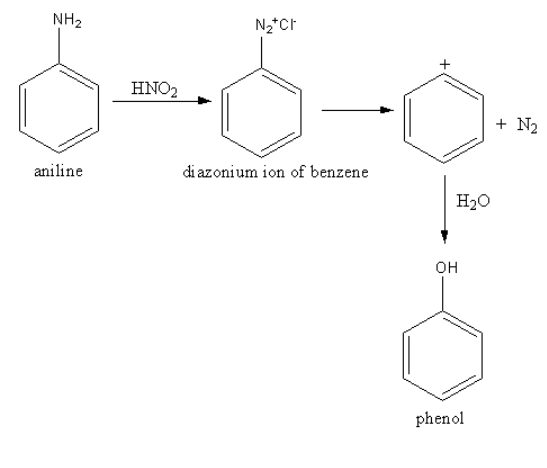
So, benzyl amine reacts with nitrous acid to form phenol mainly.
Therefore, option (A) phenol, is correct.
Additional information: The nitro compound can also convert into diazonium salts. By the reduction of nitro compounds, amine forms. The reducing agent as tin chloride in the presence of hydrochloric acid can be used for the reduction of the nitro group.
The formed diazo salt is also used for the preparation of the diazo compound. These compounds positively charged nitrogen attack on the nucleophilic centre adding two compounds by nitrogen-nitrogen double compound. These compounds are used as a dye.
Note: The mixture of sodium nitrate and hydrochloric acid is used for the preparation of nitrous acid. The reaction takes place at a low temperature. Diazonium chloride is an unstable species. The benzene cation is electrophile on which nucleophilic addition reaction takes place. Generally, the aromatic ring gives an electrophilic substitution reaction. This reaction is good for nucleophile substitution on benzene.
Complete step by step solution: Benzyl amine is also known as aniline. The reaction of benzyl amine with nitrous acid gives benzene diazonium salt. The amine group has a lone pair of electrons, so it attacks on nitrous acid. By this reaction, dinitrogen adds to the benzene ring. The formed salt is known as diazonium salt.
The dinitrogen has a positive charge. Release and form benzene cation which is an electrophile. In this electrophilic ring, the hydroxyl group (nucleophile) attacks to give phenol.
The reaction is as follows:

So, benzyl amine reacts with nitrous acid to form phenol mainly.
Therefore, option (A) phenol, is correct.
Additional information: The nitro compound can also convert into diazonium salts. By the reduction of nitro compounds, amine forms. The reducing agent as tin chloride in the presence of hydrochloric acid can be used for the reduction of the nitro group.
The formed diazo salt is also used for the preparation of the diazo compound. These compounds positively charged nitrogen attack on the nucleophilic centre adding two compounds by nitrogen-nitrogen double compound. These compounds are used as a dye.
Note: The mixture of sodium nitrate and hydrochloric acid is used for the preparation of nitrous acid. The reaction takes place at a low temperature. Diazonium chloride is an unstable species. The benzene cation is electrophile on which nucleophilic addition reaction takes place. Generally, the aromatic ring gives an electrophilic substitution reaction. This reaction is good for nucleophile substitution on benzene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




