
Both geometrical and optical isomerism are shown by:
A. ${{\left[ Co{{\left( en \right)}_{2}}C{{l}_{2}} \right]}^{+}}$
B. ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]}^{2+}}$
C. ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{4}}C{{l}_{2}} \right]}^{+}}$
D. ${{\left[ Cr{{\left( ox \right)}_{3}} \right]}^{3-}}$
Answer
573.6k+ views
Hint: We need to find the isomers of each of the compounds and check whether they fulfil the criteria of geometrical and optical isomerism. Think about the definitions of the two types of isomers.
Complete Solution :
First, we will see what geometrical and optical isomers are:
- Geometrical isomers: Molecules are said to be geometrical isomers when they have the same empirical formula but the way the atoms are arranged is different. The atom to atom bonds may be different. Geometrical isomers are usually classified as cis and trans.
- Optical isomers: Optical isomers are molecules that have the same molecular formula and the atom to atom bonds are also the same. But, the way these atoms rearranged in space is different. These isomers are non-superimposable mirror images and are called enantiomers. They are usually classified based on their optical activity as dextro or laevo rotatory.
We will look at each of the options given one by one and see what types of isomers they can form.
- The geometric isomers of ${{\left[ Co{{\left( en \right)}_{2}}C{{l}_{2}} \right]}^{+}}$ are shown below. Two isomers cis- and trans- forms are possible for this compound. The $en$ ligand denotes the ethylene diamine ligand. It is a bidentate ligand with the structural formula of $-{{H}_{2}}N-C{{H}_{2}}-C{{H}_{2}}-N{{H}_{2}}-$. Here the nitrogen atoms donate their lone pairs to form a bond. Since there are two ligands of each type available, we can say that cis and trans isomers are possible for this complex. They are as follows:
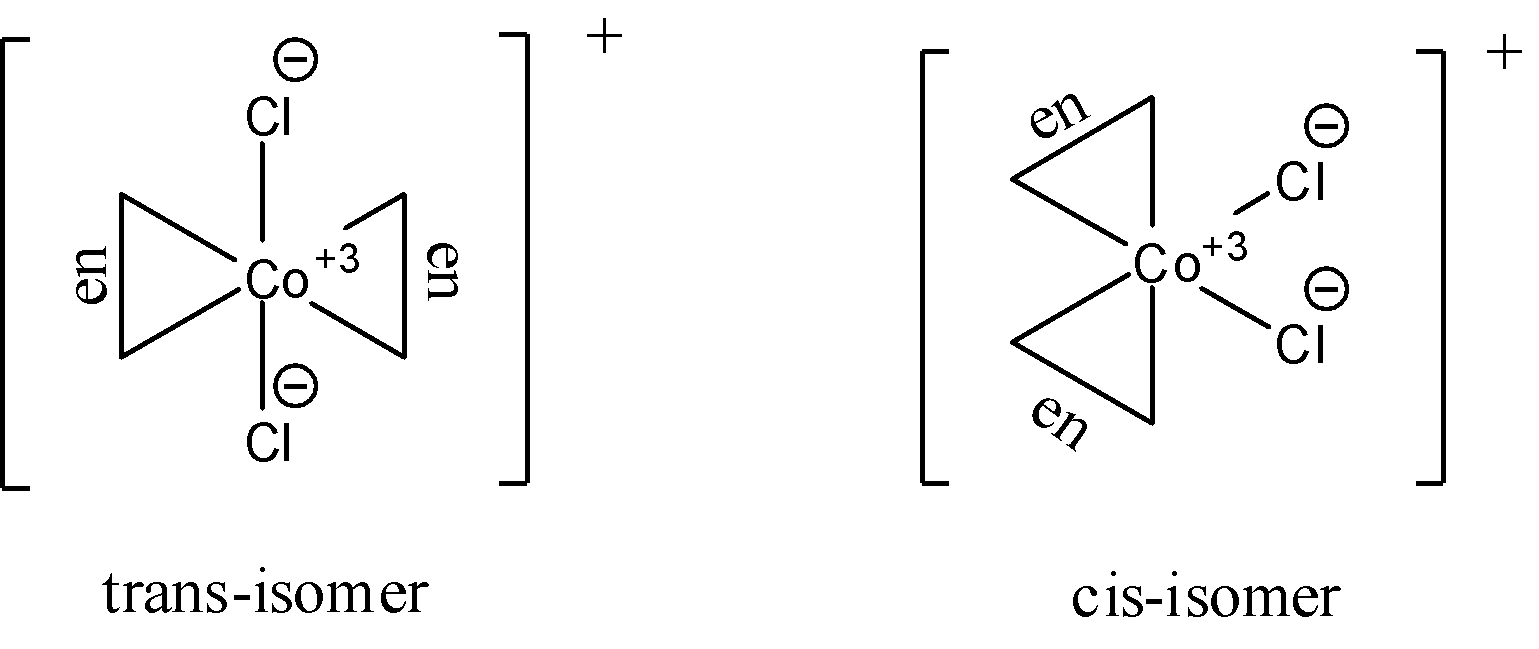
Optical isomerism of the compound is found in the cis-isomer in dextro and laevo. We know that we do not usually see optical isomerism in trans-isomers due to the existence of the plane of symmetry.
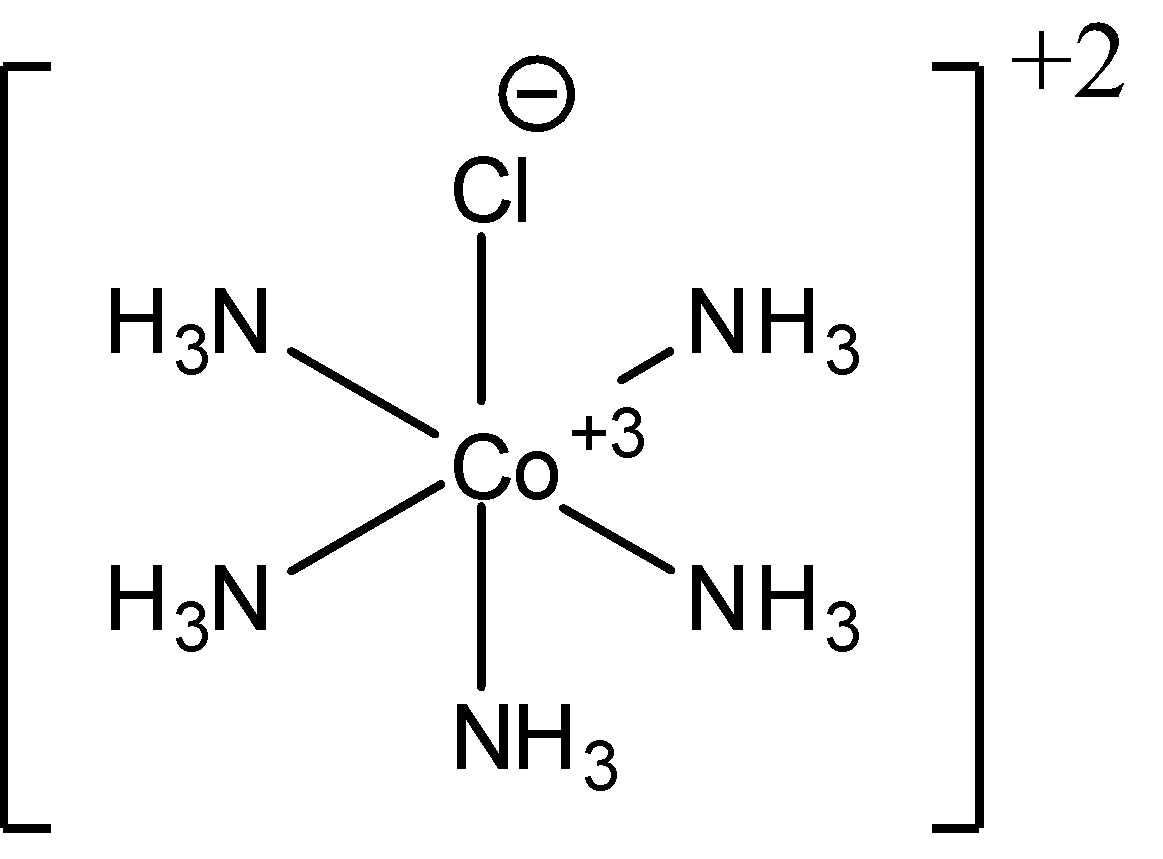
- For ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]}^{2+}}$, the type of isomerism is ionisation isomerism and geometric isomerism is not possible. Since, no matter how we arrange the ligands, one chlorine ligand is always going to lie between 2 amine ligands. This proves that no geometrical isomerism is possible. We can also see a plane of symmetry in this complex. Thus, no geometric optical isomers can be found. The structure of ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]}^{2+}}$ is as follows:
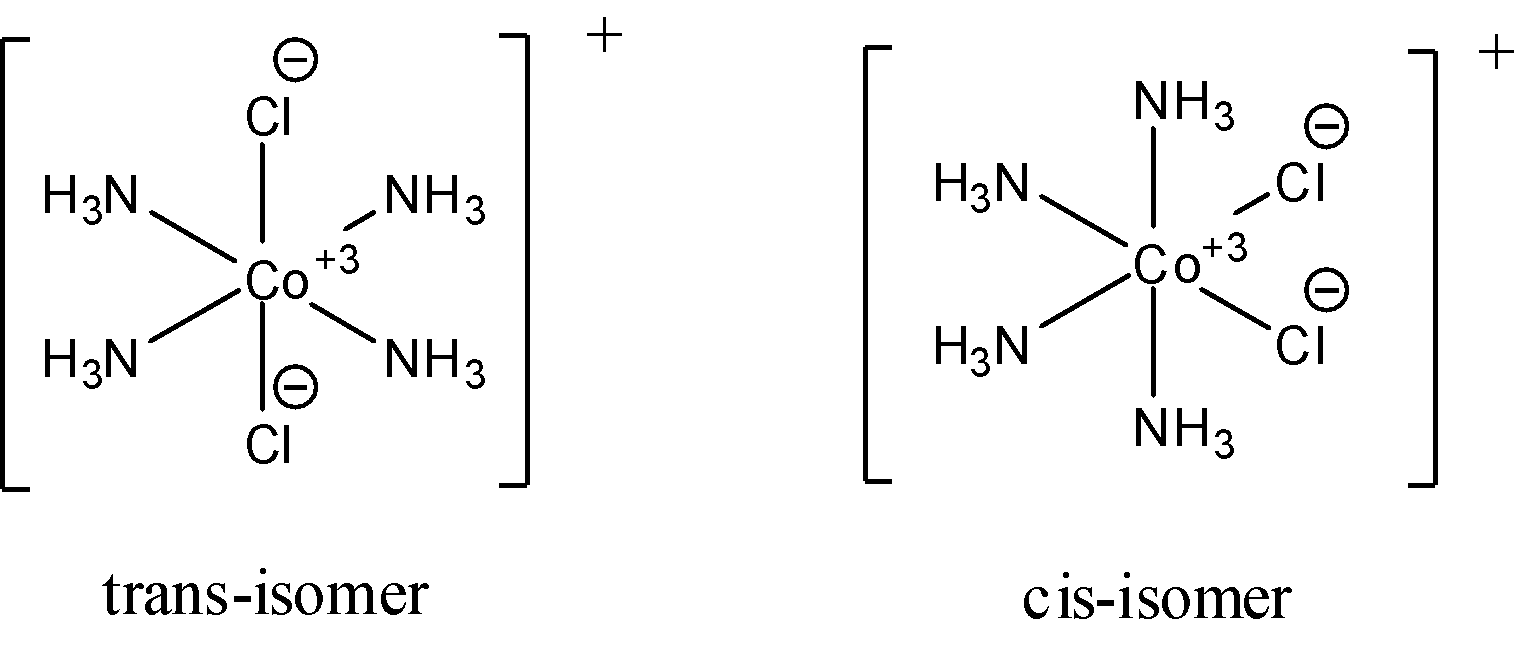
- For ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{4}}C{{l}_{2}} \right]}^{+}}$, cis and trans (geometric) isomers are possible and the cis isomer is polar while the trans isomer is non-polar. Optical isomers are not possible for this compound because it has 2 ligands occupying adjacent position to each other is called cis-isomer and the ones occupying opposite positions are called trans-isomer. Both the cis and the trans isomers have a plane of symmetry. The following diagrams show the cis and trans isomers of this compound.
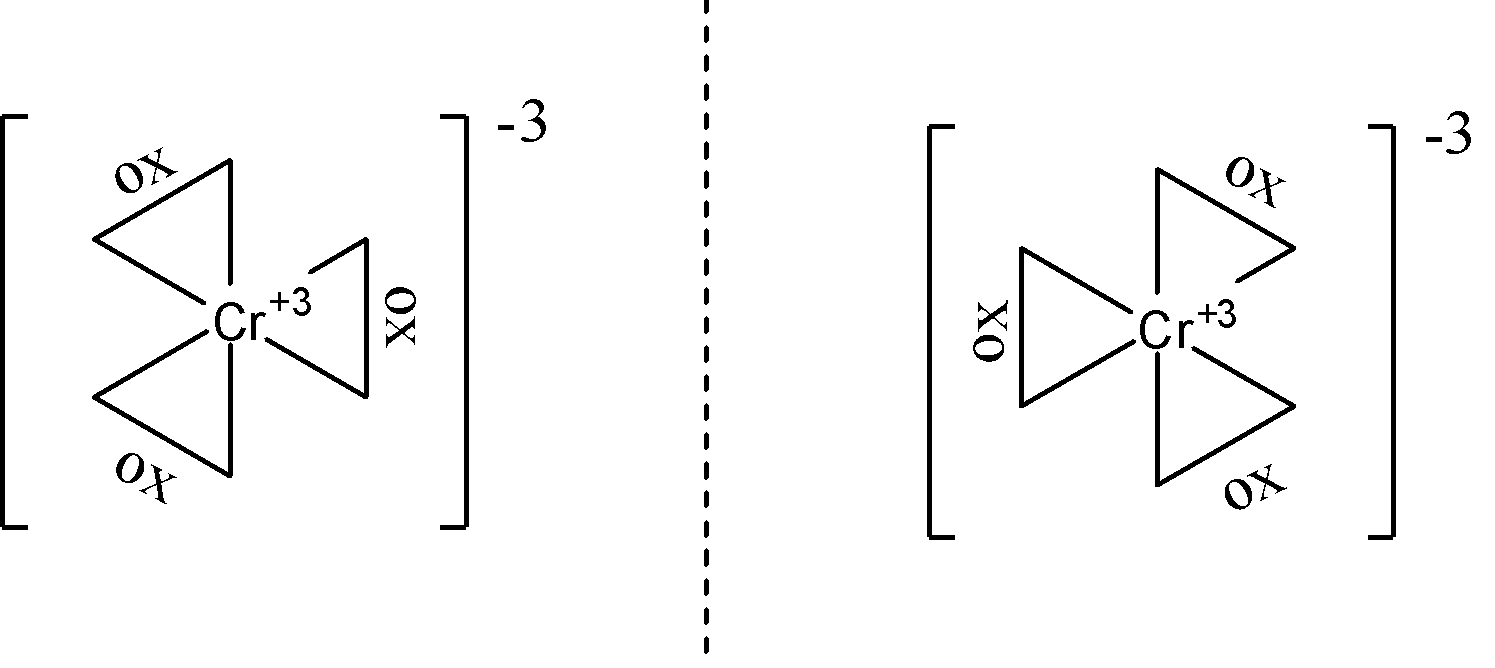
- ${{\left[ Cr{{\left( ox \right)}_{3}} \right]}^{3-}}$ does not show geometrical isomerism. We can see that all three bidentate ligands present here are the same so no matter how we arrange them, they are going to be next to each other, hence no geometric isomerism can be seen. The $ox$ ligand here is the oxalate ligand with the structural formula of $-{{O}^{-}}-CO-CO-{{O}^{-}}-$. Due to the irrotational nature of bidentate ligands, optical isomerism can be seen in this complex. The structures are as follows:
So, we come to the conclusion that the only compound that shows both geometrical and optical isomerism is ${{[Co{{(en)}_{2}}C{{l}_{2}}]}^{+}}$.
So, the correct answer is “Option A”.
Note: - To check for geometrical isomerism, we need to see the following criteria:
Two different groups on the left-hand end of the bond and two different groups on the right-hand end.
- Relative bond positions need to be taken into consideration.
- To check for optical isomerism, we need to see the following criteria:
Check for planes of symmetry
- Irrotational structures like double bonds or bidentate ligands
Complete Solution :
First, we will see what geometrical and optical isomers are:
- Geometrical isomers: Molecules are said to be geometrical isomers when they have the same empirical formula but the way the atoms are arranged is different. The atom to atom bonds may be different. Geometrical isomers are usually classified as cis and trans.
- Optical isomers: Optical isomers are molecules that have the same molecular formula and the atom to atom bonds are also the same. But, the way these atoms rearranged in space is different. These isomers are non-superimposable mirror images and are called enantiomers. They are usually classified based on their optical activity as dextro or laevo rotatory.
We will look at each of the options given one by one and see what types of isomers they can form.
- The geometric isomers of ${{\left[ Co{{\left( en \right)}_{2}}C{{l}_{2}} \right]}^{+}}$ are shown below. Two isomers cis- and trans- forms are possible for this compound. The $en$ ligand denotes the ethylene diamine ligand. It is a bidentate ligand with the structural formula of $-{{H}_{2}}N-C{{H}_{2}}-C{{H}_{2}}-N{{H}_{2}}-$. Here the nitrogen atoms donate their lone pairs to form a bond. Since there are two ligands of each type available, we can say that cis and trans isomers are possible for this complex. They are as follows:

Optical isomerism of the compound is found in the cis-isomer in dextro and laevo. We know that we do not usually see optical isomerism in trans-isomers due to the existence of the plane of symmetry.
- For ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]}^{2+}}$, the type of isomerism is ionisation isomerism and geometric isomerism is not possible. Since, no matter how we arrange the ligands, one chlorine ligand is always going to lie between 2 amine ligands. This proves that no geometrical isomerism is possible. We can also see a plane of symmetry in this complex. Thus, no geometric optical isomers can be found. The structure of ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]}^{2+}}$ is as follows:

- For ${{\left[ Co{{\left( N{{H}_{3}} \right)}_{4}}C{{l}_{2}} \right]}^{+}}$, cis and trans (geometric) isomers are possible and the cis isomer is polar while the trans isomer is non-polar. Optical isomers are not possible for this compound because it has 2 ligands occupying adjacent position to each other is called cis-isomer and the ones occupying opposite positions are called trans-isomer. Both the cis and the trans isomers have a plane of symmetry. The following diagrams show the cis and trans isomers of this compound.

- ${{\left[ Cr{{\left( ox \right)}_{3}} \right]}^{3-}}$ does not show geometrical isomerism. We can see that all three bidentate ligands present here are the same so no matter how we arrange them, they are going to be next to each other, hence no geometric isomerism can be seen. The $ox$ ligand here is the oxalate ligand with the structural formula of $-{{O}^{-}}-CO-CO-{{O}^{-}}-$. Due to the irrotational nature of bidentate ligands, optical isomerism can be seen in this complex. The structures are as follows:

So, we come to the conclusion that the only compound that shows both geometrical and optical isomerism is ${{[Co{{(en)}_{2}}C{{l}_{2}}]}^{+}}$.
So, the correct answer is “Option A”.
Note: - To check for geometrical isomerism, we need to see the following criteria:
Two different groups on the left-hand end of the bond and two different groups on the right-hand end.
- Relative bond positions need to be taken into consideration.
- To check for optical isomerism, we need to see the following criteria:
Check for planes of symmetry
- Irrotational structures like double bonds or bidentate ligands
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




