
Calcium fluoride, having the fluorite structure, the coordination number for calcium ion $(Ca_{}^{2 + })$ and fluoride ion \[\left( {{F^{ - 1}}} \right)\] are:
A. 8 and 4
B. 4 and 8
C. 4 and 2
D. 6 and 6
Answer
557.7k+ views
Hint: We know that Calcium fluoride crystallizes in a face centered cubic or CCP unit cell and has a fluorite type structure (\[1:2\]type of structure). In a fcc lattice, the atoms are present at the face centers along with the corners (lattice points), i.e., it is a non-primitive lattice. Coordination number is the number of the adjacent atoms that the reference atom is surrounded by.
We should keep this in mind that Generally anions are present at the lattice points and cations at the voids but this is not followed in calcium fluoride.
While counting the number of atoms for a lattice we only consider one-unit cells but while counting the coordination number we should consider all unit cells.
Complete step-by-step answer:Calcium fluoride is a crystalline solid. Every crystalline solid has well defined crystal structure and different compounds possess different types of crystal structure.
In Calcium fluoride, $C{a^{2 + }}$ ions form $CCP$ while ${F^ - }$ ions are in all the tetrahedral voids i.e., are present at the body diagonals.
The coordination number of $C{a^{2 + }}$ ion is eight i.e., each calcium cation is surrounded by eight fluoride anions in a body centered cubic arrangement. Each fluoride ion is in contact with four calcium ions (one calcium at the corners and the other three at the faces surrounding that body diagonal). Therefore, each unit cell has 4 calcium fluoride molecules. Solids forming fluorite type of structure are calcium fluoride, strontium fluoride, barium fluoride, barium chloride etc.
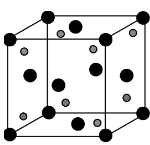
Hence, $Ca{F_2}$ has $8:4$co-ordination.
Additional information:The fluorite structure is also represented by other halides of alkaline earth metals and by ionic compounds of the general formula $A{B_2}$ like $U{O_2}$, $Th{O_2}$, $Pb{O_2}$, $Hg{F_2}$ etc.
Let ‘a’ be the edge length of the unit cell and ${r_c}$ and ${r_a}$ be the radius of $C{a^{2 + }}$ and ${F^ - }$ ions respectively, then
${r_c} + {r_a} = $$\dfrac{{\sqrt 3 \;a}}{4}$
So the correct option is (A).
Note:It may be mentioned here that sodium oxide has similar structure as that of calcium fluoride except that the positions of cations and anions are reversed i.e., oxide ion form CCP arrangement and sodium ion occupy all the tetrahedral sites. As such the coordination number of sodium ions is $4$ and that of oxide ions becomes $8$. This structure is also known as antifluorite structure. Coordination number, in these cases is opposite of molar ratio.
We should keep this in mind that Generally anions are present at the lattice points and cations at the voids but this is not followed in calcium fluoride.
While counting the number of atoms for a lattice we only consider one-unit cells but while counting the coordination number we should consider all unit cells.
Complete step-by-step answer:Calcium fluoride is a crystalline solid. Every crystalline solid has well defined crystal structure and different compounds possess different types of crystal structure.
In Calcium fluoride, $C{a^{2 + }}$ ions form $CCP$ while ${F^ - }$ ions are in all the tetrahedral voids i.e., are present at the body diagonals.
The coordination number of $C{a^{2 + }}$ ion is eight i.e., each calcium cation is surrounded by eight fluoride anions in a body centered cubic arrangement. Each fluoride ion is in contact with four calcium ions (one calcium at the corners and the other three at the faces surrounding that body diagonal). Therefore, each unit cell has 4 calcium fluoride molecules. Solids forming fluorite type of structure are calcium fluoride, strontium fluoride, barium fluoride, barium chloride etc.

Hence, $Ca{F_2}$ has $8:4$co-ordination.
Additional information:The fluorite structure is also represented by other halides of alkaline earth metals and by ionic compounds of the general formula $A{B_2}$ like $U{O_2}$, $Th{O_2}$, $Pb{O_2}$, $Hg{F_2}$ etc.
Let ‘a’ be the edge length of the unit cell and ${r_c}$ and ${r_a}$ be the radius of $C{a^{2 + }}$ and ${F^ - }$ ions respectively, then
${r_c} + {r_a} = $$\dfrac{{\sqrt 3 \;a}}{4}$
So the correct option is (A).
Note:It may be mentioned here that sodium oxide has similar structure as that of calcium fluoride except that the positions of cations and anions are reversed i.e., oxide ion form CCP arrangement and sodium ion occupy all the tetrahedral sites. As such the coordination number of sodium ions is $4$ and that of oxide ions becomes $8$. This structure is also known as antifluorite structure. Coordination number, in these cases is opposite of molar ratio.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




