
How to calculate n factor in non-redox reactions and other such reactions?
Answer
557.4k+ views
Hint: We need to remember that the valency factor or conversion factor is the n factor. The calculation of valency factor depends upon the type of reactant which we are using to find the n factor. For non-redox reactions, n factor of a substance is equal to the product of displaced mole and charge of the product.
Complete step by step answer:
We need to know that the equivalent weight of the substance is calculated by $\dfrac{\text{molecular weight}}{n}$.
This is often inferred that the no. of gram equivalents of a substance is equal to $n \times {\text{number of moles}}$ and also Normality $ = n \times molality$.
It is appropriate to use the law of gram equivalence to solve problems supporting chemical reactions. Consistent with this law, the number of gram equivalents of all reactants are adequate to the amount of gram equivalents of all products, assuming that each one the reactants are undergoing within the reaction.
We can use the above law conveniently to unravel problems without recurring to understand much about the reactions. For this we’d like to possess a good understanding of the n factor of the substance.
Non-redox reaction: It is a chemical reaction in which neither oxidation nor reduction takes place.
For Acids: Acids are the species which provides ${H^ + }$ ions when dissolved during the solvent.
For acids, the number of ${H^ + }$ ions replaced by one mole of acid during a reaction is called n factor. It is important to note that the n factor for acid isn’t adequate for its basicity, i.e. the amount of moles of replaceable ${H^ + }$ ions present in one mole or acid.
For example,
N factor of $HCl$ = $1$
N factor of $HN{O_3}$ = $1$
N factor of ${H_2}S{O_4}$ = $1$ or $2$, depending on the extent of reaction it undergoes.
${H_2}S{O_4} + NaOH \to NaHS{O_4} + {H_2}O$
In the above reaction, although one mole of ${H_2}S{O_4}$ has two replaceable hydrogen atoms but during this reaction ${H_2}S{O_4}$ has given just one ${H^ + }$ ion, so n factor for this would be $1$.
${H_2}S{O_4} + 2NaOH \to N{a_2}S{O_4} + 2{H_2}O$
The n factor for ${H_2}S{O_4}$ during the above reaction would be $2$.
Similarly,
${H_2}S{O_3}$ n factor = $1$ or $2$
${H_2}C{O_3}$ n factor = $1$ or $2$
${H_2}P{O_4}$ n factor = $1$ or $2$ or $3$
${H_3}P{O_3}$ n factor = $1$ or $2$, it is because one among the hydrogen isn’t replaceable in ${H_3}P{O_3}$. This will be seen using structure.
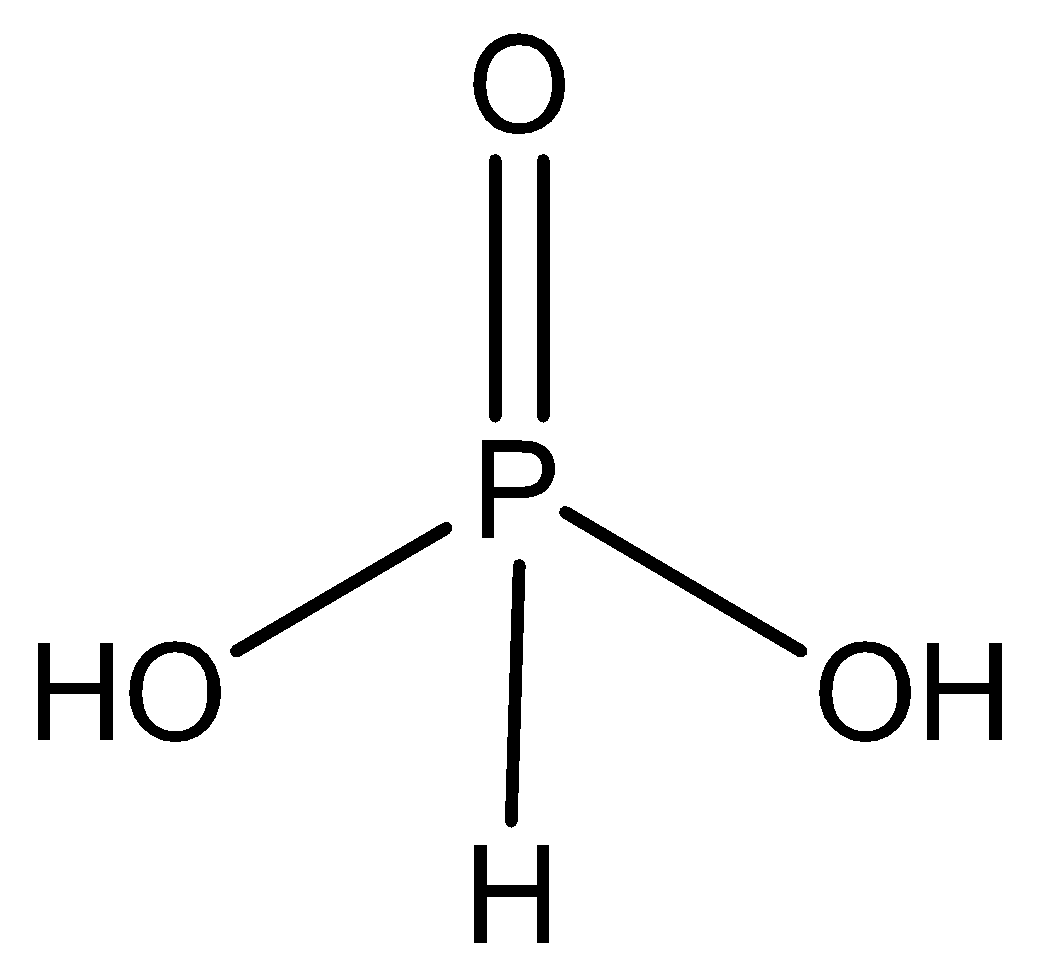
${H_3}B{O_3}$ n factor = $1$.
The three hydrogen atoms are replaceable in ${H_3}B{O_3}$ . But boron is an electron deficient atom, so it acts as a Lewis acid. When ${H_3}B{O_3}$ is reacted with water, ${H_2}O$, the oxygen atom of water attacks the boron atom, though its lone pair. The equation is follows,
${H_3}B{O_3} + 2{H_2}O \to {[B{(OH)_4}]^ - } + {H_3}{O^{_ + }}$
Thus, one mole of ${H_3}B{O_3}$ in the solution gives just one mole of ${H^ + }$, so its n factor is one.
Bases: Bases are the species that provides $O{H^ - }$ ions when dissolved during a solvent.
For bases, the number of $O{H^ - }$ ions replaced by one mole of base during a reaction is called n factor.
It is important to note that n factor isn’t adequate to its acidity, i.e. the amount of moles of replaceable $O{H^ - }$ ions present in one mole of a base.
For example,
$NaOH$ n factor = $1$
$Al{(OH)_3}$ n factor = $1$ or $2$ or $3$
$N{H_4}OH$ n factor = $1$
$Zn{(OH)_2}$ n factor = $1$ or $2$
$Ca{(OH)_2}$ n factor = $1$ or $2$
Note:
The n factor for salt is, a salt reacts such that no atom of the salt undergoes any change in oxidation number. For example,
$2AgN{O_3} + MgC{l_2} \to Mg{(N{O_3})_2}{ + _2}AgCl$
In the above reaction, it is seen that the oxidation number of $Ag,N,O,Mg$ and $Cl$ remains the same even after the reaction even within the product.
The n factor for such a salt is that the total charge on cation or anion.
Complete step by step answer:
We need to know that the equivalent weight of the substance is calculated by $\dfrac{\text{molecular weight}}{n}$.
This is often inferred that the no. of gram equivalents of a substance is equal to $n \times {\text{number of moles}}$ and also Normality $ = n \times molality$.
It is appropriate to use the law of gram equivalence to solve problems supporting chemical reactions. Consistent with this law, the number of gram equivalents of all reactants are adequate to the amount of gram equivalents of all products, assuming that each one the reactants are undergoing within the reaction.
We can use the above law conveniently to unravel problems without recurring to understand much about the reactions. For this we’d like to possess a good understanding of the n factor of the substance.
Non-redox reaction: It is a chemical reaction in which neither oxidation nor reduction takes place.
For Acids: Acids are the species which provides ${H^ + }$ ions when dissolved during the solvent.
For acids, the number of ${H^ + }$ ions replaced by one mole of acid during a reaction is called n factor. It is important to note that the n factor for acid isn’t adequate for its basicity, i.e. the amount of moles of replaceable ${H^ + }$ ions present in one mole or acid.
For example,
N factor of $HCl$ = $1$
N factor of $HN{O_3}$ = $1$
N factor of ${H_2}S{O_4}$ = $1$ or $2$, depending on the extent of reaction it undergoes.
${H_2}S{O_4} + NaOH \to NaHS{O_4} + {H_2}O$
In the above reaction, although one mole of ${H_2}S{O_4}$ has two replaceable hydrogen atoms but during this reaction ${H_2}S{O_4}$ has given just one ${H^ + }$ ion, so n factor for this would be $1$.
${H_2}S{O_4} + 2NaOH \to N{a_2}S{O_4} + 2{H_2}O$
The n factor for ${H_2}S{O_4}$ during the above reaction would be $2$.
Similarly,
${H_2}S{O_3}$ n factor = $1$ or $2$
${H_2}C{O_3}$ n factor = $1$ or $2$
${H_2}P{O_4}$ n factor = $1$ or $2$ or $3$
${H_3}P{O_3}$ n factor = $1$ or $2$, it is because one among the hydrogen isn’t replaceable in ${H_3}P{O_3}$. This will be seen using structure.

${H_3}B{O_3}$ n factor = $1$.
The three hydrogen atoms are replaceable in ${H_3}B{O_3}$ . But boron is an electron deficient atom, so it acts as a Lewis acid. When ${H_3}B{O_3}$ is reacted with water, ${H_2}O$, the oxygen atom of water attacks the boron atom, though its lone pair. The equation is follows,
${H_3}B{O_3} + 2{H_2}O \to {[B{(OH)_4}]^ - } + {H_3}{O^{_ + }}$
Thus, one mole of ${H_3}B{O_3}$ in the solution gives just one mole of ${H^ + }$, so its n factor is one.
Bases: Bases are the species that provides $O{H^ - }$ ions when dissolved during a solvent.
For bases, the number of $O{H^ - }$ ions replaced by one mole of base during a reaction is called n factor.
It is important to note that n factor isn’t adequate to its acidity, i.e. the amount of moles of replaceable $O{H^ - }$ ions present in one mole of a base.
For example,
$NaOH$ n factor = $1$
$Al{(OH)_3}$ n factor = $1$ or $2$ or $3$
$N{H_4}OH$ n factor = $1$
$Zn{(OH)_2}$ n factor = $1$ or $2$
$Ca{(OH)_2}$ n factor = $1$ or $2$
Note:
The n factor for salt is, a salt reacts such that no atom of the salt undergoes any change in oxidation number. For example,
$2AgN{O_3} + MgC{l_2} \to Mg{(N{O_3})_2}{ + _2}AgCl$
In the above reaction, it is seen that the oxidation number of $Ag,N,O,Mg$ and $Cl$ remains the same even after the reaction even within the product.
The n factor for such a salt is that the total charge on cation or anion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




