
How many carbocations given below are less stable than sec. butyl carbocation? t-butyl carbocation, Benzyl carbocation, Allyl carbocation, cyclopropenyl cation, Tropylium cation, n-butyl carbocation, n-Propyl carbocation, cyclopropyl methyl carbocation.
(A) 1
(B) 2
(C) 3
(D) 4
Answer
585.6k+ views
Hint: The carbocation is the intermediates. They are the positively charged carbon atoms. The stability carbocation depends on the inductive effect $\text{ +I }$or hyper conjugation, resonance. Some carbocation possesses extended stability due to the aromaticity.
Complete step by step answer:
A carbocation is the organic reaction intermediates. The carbocation has a positively charged carbon atom bonded to the three other atoms and has no nonbonding electrons. A carbon in a carbocation is $\text{ s}{{\text{p}}^{\text{3}}}\text{ }$ hybridized. Carbon has a planar structure with the bond angle of about
$\text{ }{{120}^{0}}\text{ }$.In carbonations, there is an empty unhybridized p orbital which is perpendicular to other orbitals.
Let's consider an example of a given carbocation.
a) n-butyl carbocation: In n-butyl carbocation, the positive charge is at the terminal carbon atom. This is a primary carbon. Therefore, the carbocation is known as the primary carbocation. The stability of carbocation increases with the addition of alkyl substituents on the carbon heading the positive charge. In n-butyl carbocation, the carbon-bearing cation is bonded and has one substituent only. Therefore, it is less stable than n-butyl carbocation.
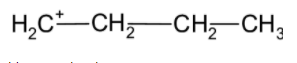
b) n-Propyl carbocation:
The n-Propyl carbocation is a follows,
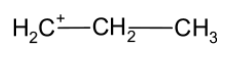
The terminal carbon of the propane bears the positive charge. It is less stable than the secondary butyl carbocation.
c) The secondary butyl carbocation is as shown below,
d) Allyl cation: In allyl cation, the positive charge is at the carbon next to the double-bonded carbon. We can say that the positively charged carbon is in conjugation with the double bond. The stability of allylic cation is found to be more than that of the sec.butyl carbocation. The allylic cation can be expressed in two resonating structures as shown below:

Thus, it exhibits higher stability than the sec.butyl carbocation.
e) tert-butyl carbocation:
It is a tertiary carbocation. The carbon-bearing the positive charge is bonded to the three methyl groups. The methyl groups have the $\text{ +I }$ effect. They donate their electron density towards the positively charged carbon and stabilize the carbocation.
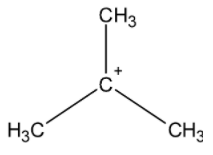
The order of stability of carbocation is as shown below:
$\text{ }{{1}^{0}}\text{ }<\text{ }{{\text{2}}^{\text{0}}}\text{ }<\text{ }{{\text{3}}^{\text{0}}}\text{ }$
Therefore, the tertiary butyl carbocation is more stable than the secondary butyl carbocation.
f) Benzyl carbocation: The benzylic cation is formed when the positive carbon atom is in conjugation with the double bond of the benzene ring. If the positive charge is on the benzylic ring, it is more likely to spread over the benzene ring. In benzylic carbocation, we can draw canonical forms. These resonating structures contribute towards the stability of the cation at the benzene ring.
The benzylic cation is as shown below,
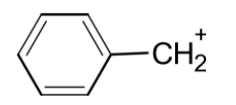
The resonating structures of it contribute towards stability. The resonating structures are as shown below,
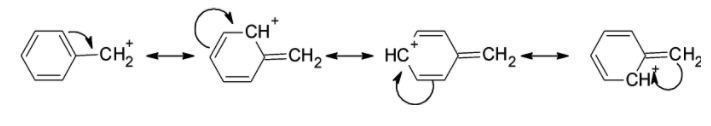
The benzyl carbocation is stable than the sec.butyl cation.
g) Cyclopropenyl cation:
Apart from the resonance, the aromaticity also provides the stability to the carbocation. Let’s consider an example of cyclopropenyl cation. The structure of it is as shown below,
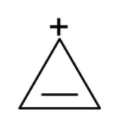
Aromaticity provides extra stability to the carbocation when the vacant p orbital is a part of the conjugated cyclic system. In cyclopropenyl cation, the double bond is in conjugation with the positive charge. The structure is cyclic and the p orbitals of the pi bond and the empty p orbital of the carbon.
The cyclopropenyl cation is more stable than the $\text{ }{{1}^{0}}\text{ }$carbonations but less stable than its aromatic analogues.
h) Tropylium cation:
The Tropylium cation also is as shown below,
It attains the extra stability due to the conjugation of positive charges with the pi bonds. It has seven resonating structures.
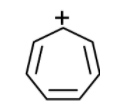
The more number of the resonating structure increases its stability concerning benzylic cation. Along with the resonance, the Tropylium ions follow the Huckle rule of aromaticity. The vacant p orbital of Tropylium ion is parallel to the p-orbitals of pi bonds.
The Tropylium ion is more stable than the benzylic cation.
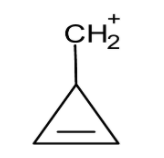
i) Cyclopropyl methyl carbocation: The cyclopropyl methyl carbocation is stable due to the conjugation of the cyclopropyl ring. The carbocation on the cyclopropyl ring is very stable compared to the cyclopropyl cation because of the conjugation of the bend orbitals of the cyclopropyl ring with the vacant p orbital of cationic carbon.
Here, there is a total of five carbocation that are more stable than the secondary butyl carbocation and two carbocation n-propyl cation and n-butyl carbocation are less stable than the secondary butyl carbocation.
Hence, (B) is the correct option.
Note: The aromatic structures obey the Huckel rule of aromaticity. The rule is stated as the structure which follows the Huckel rule has the integer value of n.
The formula is, $\text{ 4n+2}=\text{ }\!\!\pi\!\!\text{ }{{e}^{-}}\text{ }$
For example, for Tropylium cation, the total number of pi electrons is 6. The Huckel rule becomes then, $\begin{align}
& \text{ 4n+2}=6 \\
& \text{ }\therefore \text{ n = 1} \\
\end{align}$
Therefore, Tropylium cation is aromatic and gives it more stability.
Complete step by step answer:
A carbocation is the organic reaction intermediates. The carbocation has a positively charged carbon atom bonded to the three other atoms and has no nonbonding electrons. A carbon in a carbocation is $\text{ s}{{\text{p}}^{\text{3}}}\text{ }$ hybridized. Carbon has a planar structure with the bond angle of about
$\text{ }{{120}^{0}}\text{ }$.In carbonations, there is an empty unhybridized p orbital which is perpendicular to other orbitals.
Let's consider an example of a given carbocation.
a) n-butyl carbocation: In n-butyl carbocation, the positive charge is at the terminal carbon atom. This is a primary carbon. Therefore, the carbocation is known as the primary carbocation. The stability of carbocation increases with the addition of alkyl substituents on the carbon heading the positive charge. In n-butyl carbocation, the carbon-bearing cation is bonded and has one substituent only. Therefore, it is less stable than n-butyl carbocation.
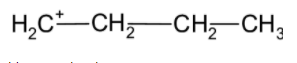
b) n-Propyl carbocation:
The n-Propyl carbocation is a follows,
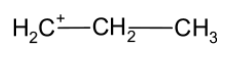
The terminal carbon of the propane bears the positive charge. It is less stable than the secondary butyl carbocation.
c) The secondary butyl carbocation is as shown below,
d) Allyl cation: In allyl cation, the positive charge is at the carbon next to the double-bonded carbon. We can say that the positively charged carbon is in conjugation with the double bond. The stability of allylic cation is found to be more than that of the sec.butyl carbocation. The allylic cation can be expressed in two resonating structures as shown below:

Thus, it exhibits higher stability than the sec.butyl carbocation.
e) tert-butyl carbocation:
It is a tertiary carbocation. The carbon-bearing the positive charge is bonded to the three methyl groups. The methyl groups have the $\text{ +I }$ effect. They donate their electron density towards the positively charged carbon and stabilize the carbocation.

The order of stability of carbocation is as shown below:
$\text{ }{{1}^{0}}\text{ }<\text{ }{{\text{2}}^{\text{0}}}\text{ }<\text{ }{{\text{3}}^{\text{0}}}\text{ }$
Therefore, the tertiary butyl carbocation is more stable than the secondary butyl carbocation.
f) Benzyl carbocation: The benzylic cation is formed when the positive carbon atom is in conjugation with the double bond of the benzene ring. If the positive charge is on the benzylic ring, it is more likely to spread over the benzene ring. In benzylic carbocation, we can draw canonical forms. These resonating structures contribute towards the stability of the cation at the benzene ring.
The benzylic cation is as shown below,

The resonating structures of it contribute towards stability. The resonating structures are as shown below,

The benzyl carbocation is stable than the sec.butyl cation.
g) Cyclopropenyl cation:
Apart from the resonance, the aromaticity also provides the stability to the carbocation. Let’s consider an example of cyclopropenyl cation. The structure of it is as shown below,

Aromaticity provides extra stability to the carbocation when the vacant p orbital is a part of the conjugated cyclic system. In cyclopropenyl cation, the double bond is in conjugation with the positive charge. The structure is cyclic and the p orbitals of the pi bond and the empty p orbital of the carbon.
The cyclopropenyl cation is more stable than the $\text{ }{{1}^{0}}\text{ }$carbonations but less stable than its aromatic analogues.
h) Tropylium cation:
The Tropylium cation also is as shown below,
It attains the extra stability due to the conjugation of positive charges with the pi bonds. It has seven resonating structures.

The more number of the resonating structure increases its stability concerning benzylic cation. Along with the resonance, the Tropylium ions follow the Huckle rule of aromaticity. The vacant p orbital of Tropylium ion is parallel to the p-orbitals of pi bonds.
The Tropylium ion is more stable than the benzylic cation.
i) Cyclopropyl methyl carbocation: The cyclopropyl methyl carbocation is stable due to the conjugation of the cyclopropyl ring. The carbocation on the cyclopropyl ring is very stable compared to the cyclopropyl cation because of the conjugation of the bend orbitals of the cyclopropyl ring with the vacant p orbital of cationic carbon.

Here, there is a total of five carbocation that are more stable than the secondary butyl carbocation and two carbocation n-propyl cation and n-butyl carbocation are less stable than the secondary butyl carbocation.
Hence, (B) is the correct option.
Note: The aromatic structures obey the Huckel rule of aromaticity. The rule is stated as the structure which follows the Huckel rule has the integer value of n.
The formula is, $\text{ 4n+2}=\text{ }\!\!\pi\!\!\text{ }{{e}^{-}}\text{ }$
For example, for Tropylium cation, the total number of pi electrons is 6. The Huckel rule becomes then, $\begin{align}
& \text{ 4n+2}=6 \\
& \text{ }\therefore \text{ n = 1} \\
\end{align}$
Therefore, Tropylium cation is aromatic and gives it more stability.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




