
Carbonyl compounds react with phenyl hydrazine to form:
A.Oxime
B.Phenyl hydrazone
C.Hydrazone
D.Semicarbazone
Answer
512.4k+ views
Hint: Carbonyl groups are those groups which contain aldehydes or ketones. Here, the carbon atom is bonded to a carbon atom and a hydrogen atom. These are generally prepared by the oxidation of alcohols and therefore, when they are reduced, they give their respective primary alcohols as a product.
Complete answer:
Complete step-by-step answer:
When carbonyl compounds like aldehydes or ketones react with phenyl hydrazine then, the following reaction takes place:
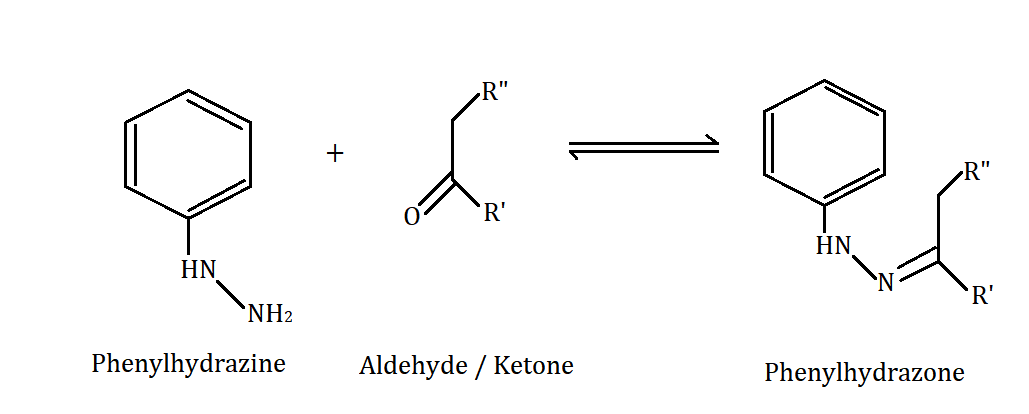
Hence, phenylhydrazone is formed on reaction.
Therefore, option B is correct.
Additional information:
Simpler aldehydes are soluble in water because of their tendency to form hydrogen bonds with water molecules. However, this property decreases as the length of the chain of the compound increases. That is why complex aldehydes are insoluble in water but are soluble in organic solvents like benzene.
To get phenylhydrazone, industries use acetaldehyde and due to this, phenylhydrazone is also sometimes called aldehyde phenylhydrazine. Acetaldehyde is used on a very large scale in industries as it is highly reactive in nature. It is also a toxic compound which is found in alcohol products and the consumption of these alcohols damages our liver functions.
Phenylhydrazine decomposes slowly to give ammonia, nitrogen and benzene. In the presence of oxygen, it burns exothermically and liberates a lot of hot gases.
Note:
Phenyl hydrazine is used to convert various sugar mixtures to phenylhydrazones. Aldehydes and ketones undergo many reactions due to the acidic nature of \[\alpha - \]hydrogen present in their compound. This acidity of \[\alpha - \]hydrogen is due to the strong electron withdrawing effect of the carbonyl group and the resonance effect of the conjugate base which makes it stable.
Complete answer:
Complete step-by-step answer:
When carbonyl compounds like aldehydes or ketones react with phenyl hydrazine then, the following reaction takes place:

Hence, phenylhydrazone is formed on reaction.
Therefore, option B is correct.
Additional information:
Simpler aldehydes are soluble in water because of their tendency to form hydrogen bonds with water molecules. However, this property decreases as the length of the chain of the compound increases. That is why complex aldehydes are insoluble in water but are soluble in organic solvents like benzene.
To get phenylhydrazone, industries use acetaldehyde and due to this, phenylhydrazone is also sometimes called aldehyde phenylhydrazine. Acetaldehyde is used on a very large scale in industries as it is highly reactive in nature. It is also a toxic compound which is found in alcohol products and the consumption of these alcohols damages our liver functions.
Phenylhydrazine decomposes slowly to give ammonia, nitrogen and benzene. In the presence of oxygen, it burns exothermically and liberates a lot of hot gases.
Note:
Phenyl hydrazine is used to convert various sugar mixtures to phenylhydrazones. Aldehydes and ketones undergo many reactions due to the acidic nature of \[\alpha - \]hydrogen present in their compound. This acidity of \[\alpha - \]hydrogen is due to the strong electron withdrawing effect of the carbonyl group and the resonance effect of the conjugate base which makes it stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




