
Why \[C{H_4}\] cannot adopt square planar geometry?
Answer
589.2k+ views
Hint: Any central atom having four atoms attached to it, can arrange in two shapes, square planar and tetrahedral on the basis of repulsion between those atoms, or lone pair on the central atom and the atom. Methane is a molecule with a carbon central atom and four hydrogen atoms attached to it.
Complete step-by-step answer:
Carbon is an element that belongs to group 13 of periodic table and has atomic number 6.
The Ground state electronic configuration of carbon is \[1{s^2}2{s^2}2p_x^12p_y^1\].
Excited state electronic configuration of carbon is \[1{s^2}2{s^1}2p_x^12p_y^12p_z^1\].
As four vacant orbitals can occupy four hydrogen atoms and form \[s{p^3}\] hybridised molecule,
commonly known as methane. \[s{p^3}\] hybridised molecules are tetrahedral in geometry and so is methane.
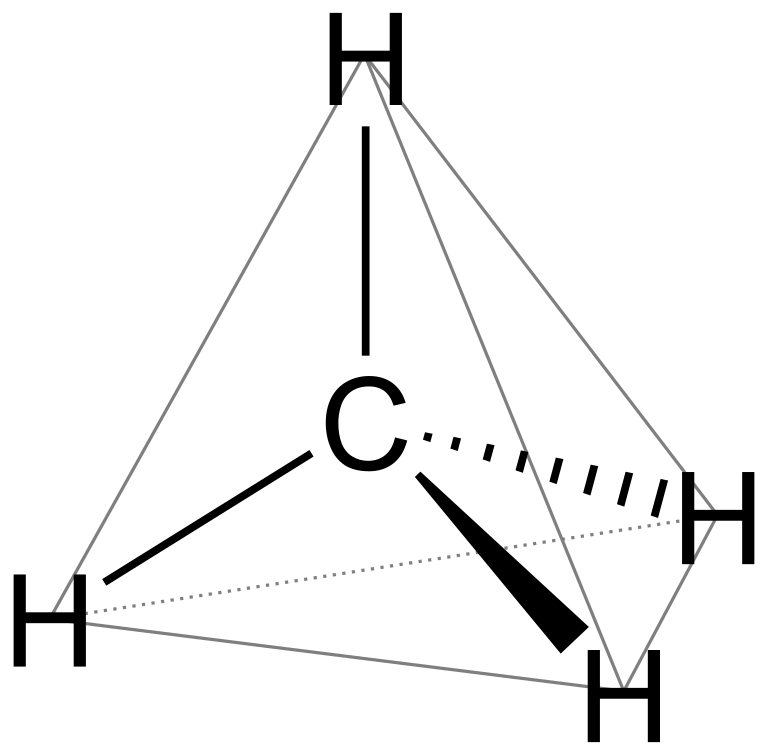
For square planar geometry, the hybridisation has to be \[ds{p^2}\]. But in methane, there is no involvement of d-orbitals as it has less electrons. So, it cannot have square planar geometry.
As per valence shell electron pair repulsion theory (VSEPR), we can't determine the structure of any molecule with the help of its hybridisation.
Therefore, we say that \[C{H_4}\] cannot adopt square planar geometry.
Note: Methane molecule is tetrahedral with \[{109.5^ \circ }\] bond angle (H-C-H), although the \[2p\] orbitals are all at $90^0$ to each other and four equal bond lengths with no dipole moment because the electronegativity difference between carbon and hydrogen is very less.
Complete step-by-step answer:
Carbon is an element that belongs to group 13 of periodic table and has atomic number 6.
The Ground state electronic configuration of carbon is \[1{s^2}2{s^2}2p_x^12p_y^1\].
Excited state electronic configuration of carbon is \[1{s^2}2{s^1}2p_x^12p_y^12p_z^1\].
As four vacant orbitals can occupy four hydrogen atoms and form \[s{p^3}\] hybridised molecule,
commonly known as methane. \[s{p^3}\] hybridised molecules are tetrahedral in geometry and so is methane.

For square planar geometry, the hybridisation has to be \[ds{p^2}\]. But in methane, there is no involvement of d-orbitals as it has less electrons. So, it cannot have square planar geometry.
As per valence shell electron pair repulsion theory (VSEPR), we can't determine the structure of any molecule with the help of its hybridisation.
Therefore, we say that \[C{H_4}\] cannot adopt square planar geometry.
Note: Methane molecule is tetrahedral with \[{109.5^ \circ }\] bond angle (H-C-H), although the \[2p\] orbitals are all at $90^0$ to each other and four equal bond lengths with no dipole moment because the electronegativity difference between carbon and hydrogen is very less.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




