
Chains, branches, and rings.
Answer
558.6k+ views
Hint: Carbon atoms form different kinds of compounds with different arrangement, such as chain, branched chain and cyclic chain structures.
Complete step by step answer:
- A hydrocarbon is an organic compound that is made up of only carbon and hydrogen. It is the simplest kind of organic molecule and is the basis for all other more complex organic compounds.
- Structural isomerism is a property of hydrocarbons showing isomerism due to difference in their structures. These compounds are referred to as structural isomers.
- Let us take Butane as an example.
The molecular formula of butane is ${{C}_{4}}{{H}_{10}}$
The structural formula of butane is illustrated in the figure shown below:
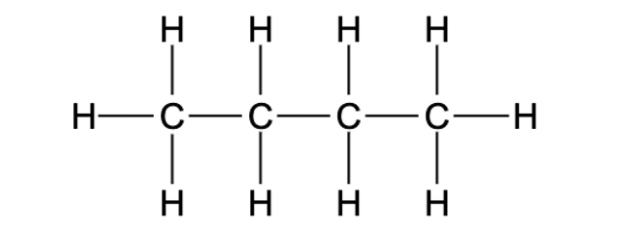
The name of this structure of butane is n-Butane. Here, four carbon atoms can be arranged in different ways. One other example is shown below:
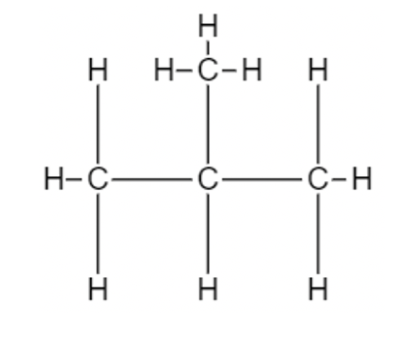
The name of this hydrocarbon is isobutane. And in this, the carbon atom is arranged as a branched chain.
- When these carbon atoms form chains in the form of rings, such hydrocarbon is known as cyclic hydrocarbon.
- Let us consider the example of cyclohexane.
The molecular formula of cyclohexane is ${{C}_{6}}{{H}_{12}}$
The structural formula is shown below:

Additional Information . Isomers are different compounds. They show different properties like different melting and boiling point.
Note: Hydrocarbons having the same numbers of atoms but different shapes form different compounds are called isomers. Hydrocarbons with 4 or more than 4 carbon atoms have isomers.
Complete step by step answer:
- A hydrocarbon is an organic compound that is made up of only carbon and hydrogen. It is the simplest kind of organic molecule and is the basis for all other more complex organic compounds.
- Structural isomerism is a property of hydrocarbons showing isomerism due to difference in their structures. These compounds are referred to as structural isomers.
- Let us take Butane as an example.
The molecular formula of butane is ${{C}_{4}}{{H}_{10}}$
The structural formula of butane is illustrated in the figure shown below:

The name of this structure of butane is n-Butane. Here, four carbon atoms can be arranged in different ways. One other example is shown below:

The name of this hydrocarbon is isobutane. And in this, the carbon atom is arranged as a branched chain.
- When these carbon atoms form chains in the form of rings, such hydrocarbon is known as cyclic hydrocarbon.
- Let us consider the example of cyclohexane.
The molecular formula of cyclohexane is ${{C}_{6}}{{H}_{12}}$
The structural formula is shown below:

Additional Information . Isomers are different compounds. They show different properties like different melting and boiling point.
Note: Hydrocarbons having the same numbers of atoms but different shapes form different compounds are called isomers. Hydrocarbons with 4 or more than 4 carbon atoms have isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




