
How many chiral centers are present in this molecule?
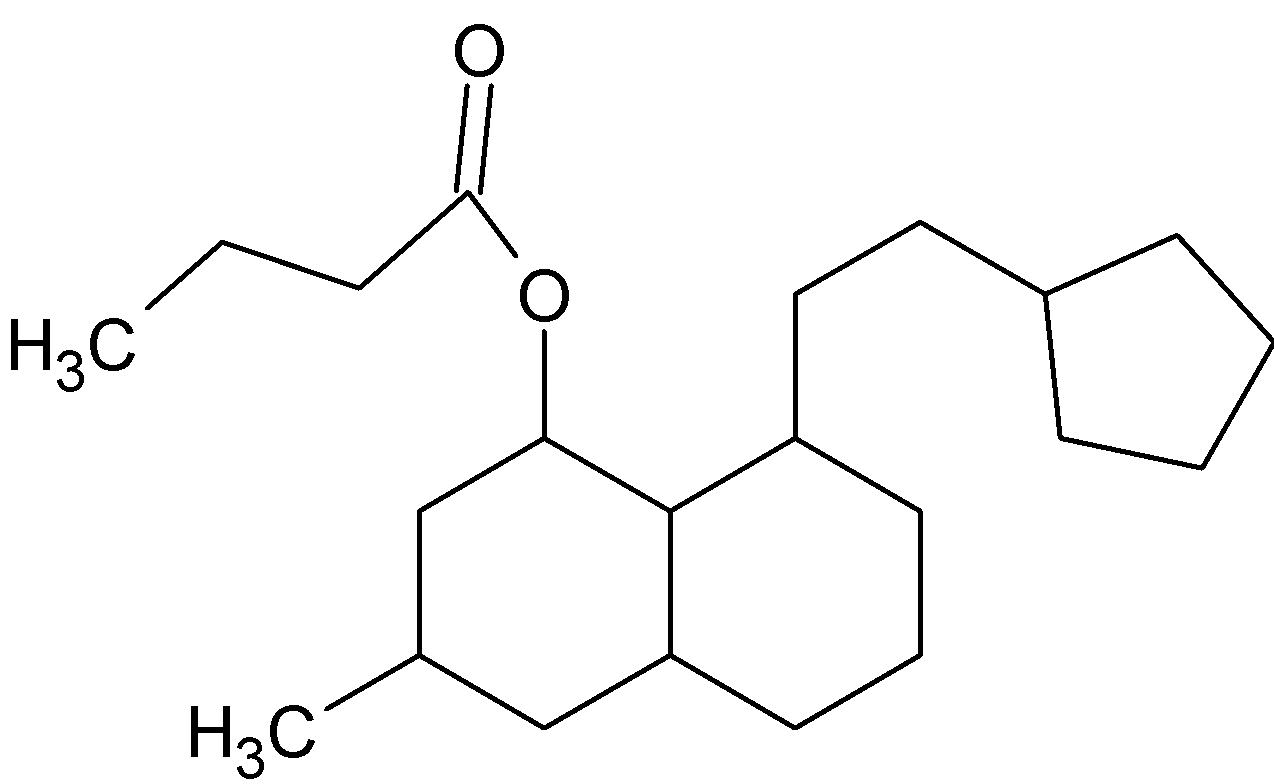

Answer
582.9k+ views
Hint: A molecule is said to be chiral if it is not superimposable on its mirror images. Optical activity is the ability of a chiral molecule to rotate the plane polarized light. Chirality often leads to optical activity in a compound, but it is not a necessary condition for optical activity.
Complete step by step answer:
Chirality is not just reserved for objects. Molecules can also be chiral. Chiral molecules are the molecules having an asymmetrical center. An asymmetric center means that a tetrahedral atom bonded to four different groups or atoms.
Chiral objects are objects with left handed and right handed forms. Non-superimposable mirror image is a mirror image that is not the same as the image itself. Chiral objects have non-superimposable mirror images.
A chiral center is a point in a molecule where four different groups are bonded. If two groups are the same, then it is not chiral. A chiral molecule usually has at least one chiral center.
For example, in a compound named bromo chloro fluoro iodo methane. The chiral center is at the carbon atom. It is bonded to four different atoms, i.e. bromine, chlorine, fluorine and iodine.
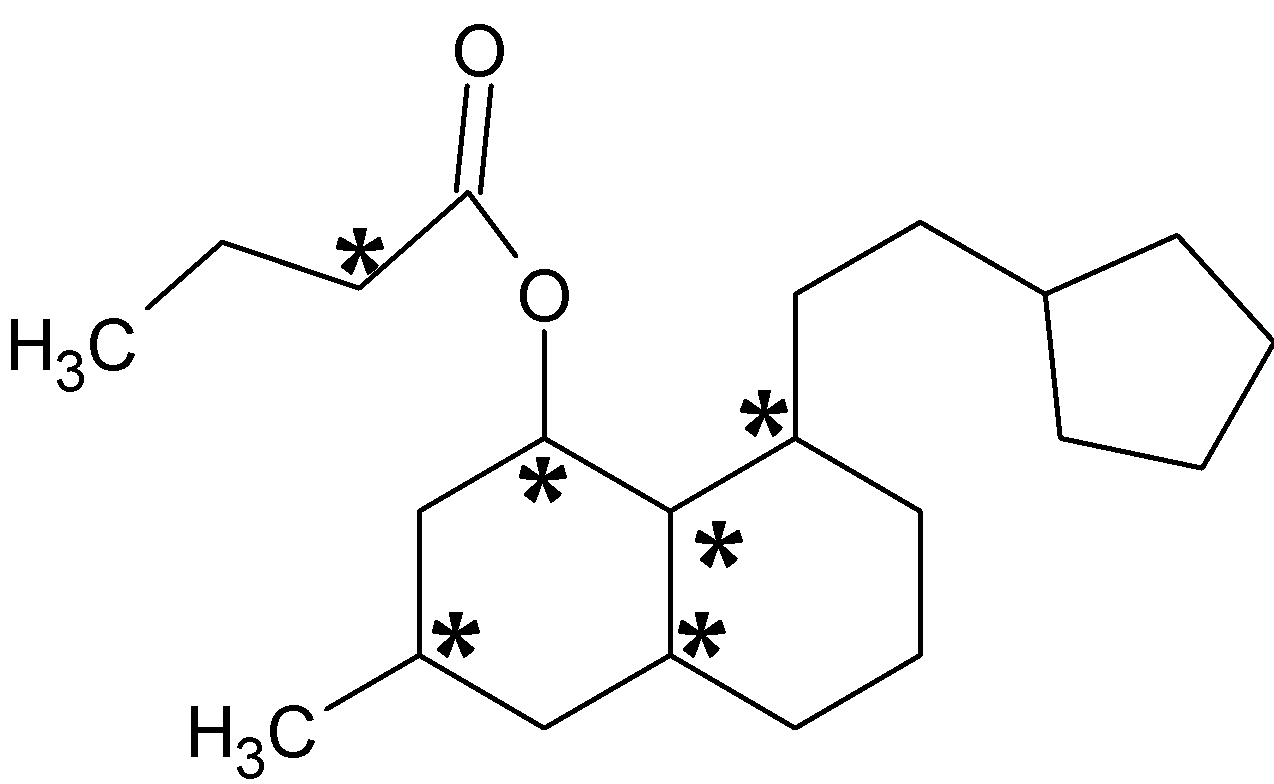
Chiral centers are mark with an asterisk (*). There are six chiral centers which are bonded to four different groups.
Note:
Chiral centers are also known as stereogenic centers. When the mirror image of an achiral carbon is rotated, and the structure can be aligned with each other, their mirror images are said to be achiral. Fischer projection is a two dimensional representation of a three dimensional molecule. This is more helpful for checking whether there is a chiral carbon atom or not.
Complete step by step answer:
Chirality is not just reserved for objects. Molecules can also be chiral. Chiral molecules are the molecules having an asymmetrical center. An asymmetric center means that a tetrahedral atom bonded to four different groups or atoms.
Chiral objects are objects with left handed and right handed forms. Non-superimposable mirror image is a mirror image that is not the same as the image itself. Chiral objects have non-superimposable mirror images.
A chiral center is a point in a molecule where four different groups are bonded. If two groups are the same, then it is not chiral. A chiral molecule usually has at least one chiral center.
For example, in a compound named bromo chloro fluoro iodo methane. The chiral center is at the carbon atom. It is bonded to four different atoms, i.e. bromine, chlorine, fluorine and iodine.

Chiral centers are mark with an asterisk (*). There are six chiral centers which are bonded to four different groups.
Note:
Chiral centers are also known as stereogenic centers. When the mirror image of an achiral carbon is rotated, and the structure can be aligned with each other, their mirror images are said to be achiral. Fischer projection is a two dimensional representation of a three dimensional molecule. This is more helpful for checking whether there is a chiral carbon atom or not.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




