
Choose the correct statement about I, II and III.
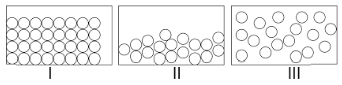
(A) I and II have definite volume but III does not have this property.
(B) I, II and III are interconvertible by changing the conditions of temperature and pressure.
(C) In the particles of I, freedom of movement is large.
(D) Both A and B.

Answer
558.9k+ views
Hint: Here, I is solid, II is liquid and III is gas, so, now we have to find out the different properties of all three states of matter and their comparisons on the different properties like definite volume, freedom of movement etc.
Complete answer:Here we discuss in the different physical properties of the different states of matter:So, we start with the properties of solids:
(a) Definite mass, volume and shape: The solids are having definite shape, volume and mass because of their high intermolecular force between the constituents.
(b) Having high density.
(c) Incompressible and rigid: They are incompressible and rigid.
(d) Solids remain fixed at their positions having freedom of movement is low.
Now talking about the properties of liquids,
(a) Definite volume: The liquids are having definite volume.
(b) Having low density and indefinite shape.
(c) Compressible: They are compressible to some extinct.
(d) Liquids having freedom of movement is lower than gases and higher than the solids.
Now talking about the properties of gas,
(a) Indefinite mass, volume and shape: The gases are having indefinite shape, volume and mass because of their very low intermolecular force between the constituents.
(b) Having very low density.
(c) Highly compressible.
(d) Gases remain moving, having freedom of movement very high.
As here, I is solid, II is liquid and III is gas.
From the above discussions, solid and liquid have definite volume but gas does not have this property and also solid, liquid and gas are interconvertible by changing the conditions of temperature and pressure, but in the particles of solid, freedom of movement is not large.
Hence, the correct option in these questions is (D) Both A and B.
Note:The liquids and gases are having slightly similar properties like they are compressible, both are having tendency to flow, they don’t have definite shape. So, they are sometimes categorised together named as fluids.
Complete answer:Here we discuss in the different physical properties of the different states of matter:So, we start with the properties of solids:
(a) Definite mass, volume and shape: The solids are having definite shape, volume and mass because of their high intermolecular force between the constituents.
(b) Having high density.
(c) Incompressible and rigid: They are incompressible and rigid.
(d) Solids remain fixed at their positions having freedom of movement is low.
Now talking about the properties of liquids,
(a) Definite volume: The liquids are having definite volume.
(b) Having low density and indefinite shape.
(c) Compressible: They are compressible to some extinct.
(d) Liquids having freedom of movement is lower than gases and higher than the solids.
Now talking about the properties of gas,
(a) Indefinite mass, volume and shape: The gases are having indefinite shape, volume and mass because of their very low intermolecular force between the constituents.
(b) Having very low density.
(c) Highly compressible.
(d) Gases remain moving, having freedom of movement very high.
As here, I is solid, II is liquid and III is gas.
From the above discussions, solid and liquid have definite volume but gas does not have this property and also solid, liquid and gas are interconvertible by changing the conditions of temperature and pressure, but in the particles of solid, freedom of movement is not large.
Hence, the correct option in these questions is (D) Both A and B.
Note:The liquids and gases are having slightly similar properties like they are compressible, both are having tendency to flow, they don’t have definite shape. So, they are sometimes categorised together named as fluids.
Recently Updated Pages
Master Class 7 English: Engaging Questions & Answers for Success

Master Class 7 Maths: Engaging Questions & Answers for Success

Master Class 7 Science: Engaging Questions & Answers for Success

Class 7 Question and Answer - Your Ultimate Solutions Guide

Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Trending doubts
The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

Convert 200 Million dollars in rupees class 7 maths CBSE

List of coprime numbers from 1 to 100 class 7 maths CBSE

AIM To prepare stained temporary mount of onion peel class 7 biology CBSE

The plural of Chief is Chieves A True B False class 7 english CBSE

Write a letter to the editor of the national daily class 7 english CBSE





