
Chromite ore has the formula:
(a)- $FeC{{r}_{2}}{{O}_{6}}$
(b)- $FeC{{r}_{2}}{{O}_{4}}$
(c)- $FeC{{r}_{2}}{{O}_{7}}$
(d)- Both A and B
Answer
589.2k+ views
Hint: Chromite is an ore from which the metals iron and chromium are commercially extracted. The chromite ore is the oxide form of iron and chromium.
Complete step by step answer:
Chromite is the ore or mineral in which the chromium metal is abundantly found. The formula of chromite is $FeC{{r}_{2}}{{O}_{4}}$. It is also called iron chromium oxide. The iron in the chromite can be replaced with the magnesium metal, which forms magnesiochromite and the formula of magnesiochromite is $MgC{{r}_{2}}{{O}_{4}}$.
The structure of chromite is:
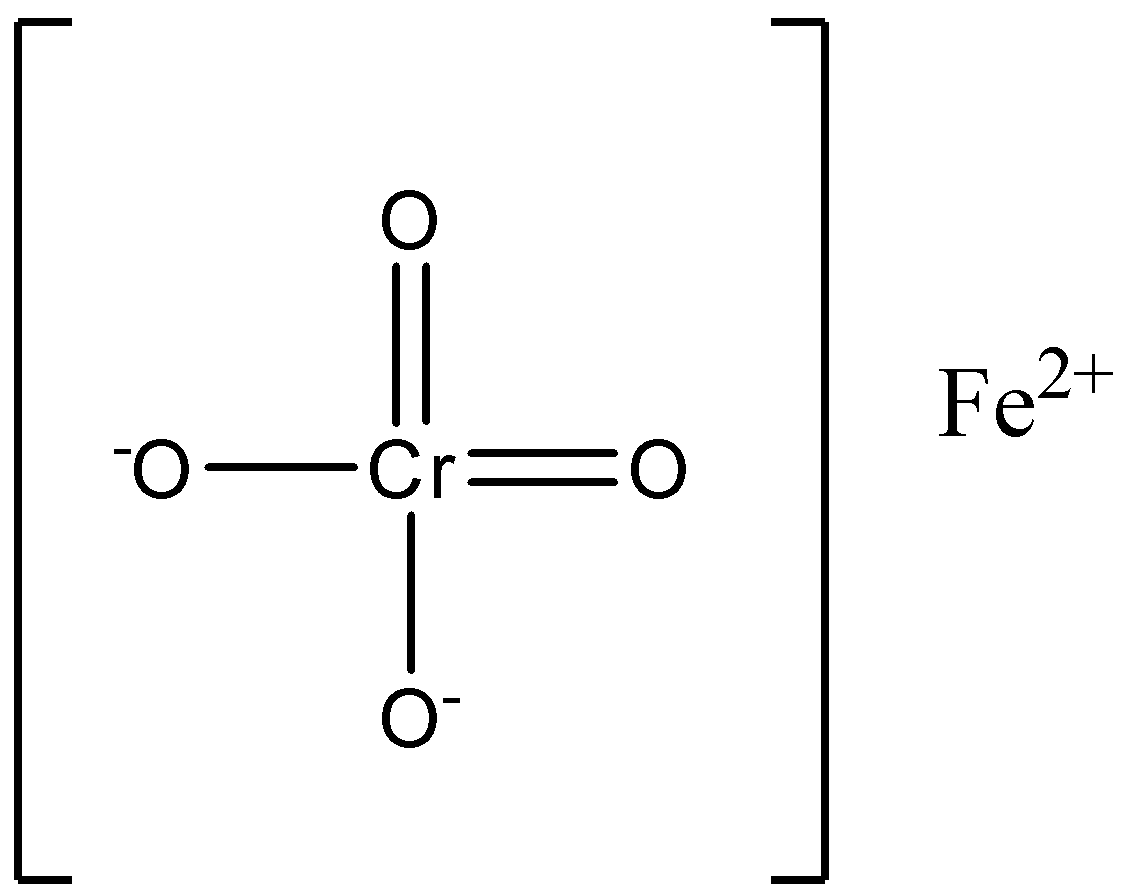
Nowadays, stainless steel is particularly made from the chromite ore. Chromite ore is commonly found in countries like South Africa and India.
Chromite ore has a metallic luster and has an iron-black color. The hardness of chromite ore on the Mohs scale is 5.5.
In the chromite formula, the iron is in the +2 oxidation state, and chromium is in the +3 oxidation state. The chromite ore is a very giant ore which is then crushed to granules.
Magnesium, iron, aluminium, and small traces of titanium are found in the chromite ore. Chromite ore belongs to the spinel group (it forms a complete solid solution series with other elements in the same group).
Chromite ore can be used to make alloys like ferroalloy. Ferrochrome is an alloy that is formed between iron and chromium, which is made from the chromite ore.
So the correct answer is an option (b)- $FeC{{r}_{2}}{{O}_{4}}$.
Note: The chromite ore has very heat stability and because of this property it is used to make refractory materials. When the chromite is mixed with iron and nickel, it forms an alloy nichrome.
Complete step by step answer:
Chromite is the ore or mineral in which the chromium metal is abundantly found. The formula of chromite is $FeC{{r}_{2}}{{O}_{4}}$. It is also called iron chromium oxide. The iron in the chromite can be replaced with the magnesium metal, which forms magnesiochromite and the formula of magnesiochromite is $MgC{{r}_{2}}{{O}_{4}}$.
The structure of chromite is:

Nowadays, stainless steel is particularly made from the chromite ore. Chromite ore is commonly found in countries like South Africa and India.
Chromite ore has a metallic luster and has an iron-black color. The hardness of chromite ore on the Mohs scale is 5.5.
In the chromite formula, the iron is in the +2 oxidation state, and chromium is in the +3 oxidation state. The chromite ore is a very giant ore which is then crushed to granules.
Magnesium, iron, aluminium, and small traces of titanium are found in the chromite ore. Chromite ore belongs to the spinel group (it forms a complete solid solution series with other elements in the same group).
Chromite ore can be used to make alloys like ferroalloy. Ferrochrome is an alloy that is formed between iron and chromium, which is made from the chromite ore.
So the correct answer is an option (b)- $FeC{{r}_{2}}{{O}_{4}}$.
Note: The chromite ore has very heat stability and because of this property it is used to make refractory materials. When the chromite is mixed with iron and nickel, it forms an alloy nichrome.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




