
What is the colour of methyl orange in acidic and basic medium respectively?
(A) Yellow, Red
(B) Red, Yellow
(C) Colourless, Red
(D) Colourless, Yellow
Answer
232.8k+ views
Hint: Methyl orange is an organic compound which is used as a laboratory agent. It is mostly used as a pH indicator frequently used in titrations due to its distinct colour and variance at different pH values.
Complete step by step answer: Methyl orange is a chemical compound having chemical formula ${C}_{14}{H}_{14}{N}_{3}Na{O}_{3}S$.
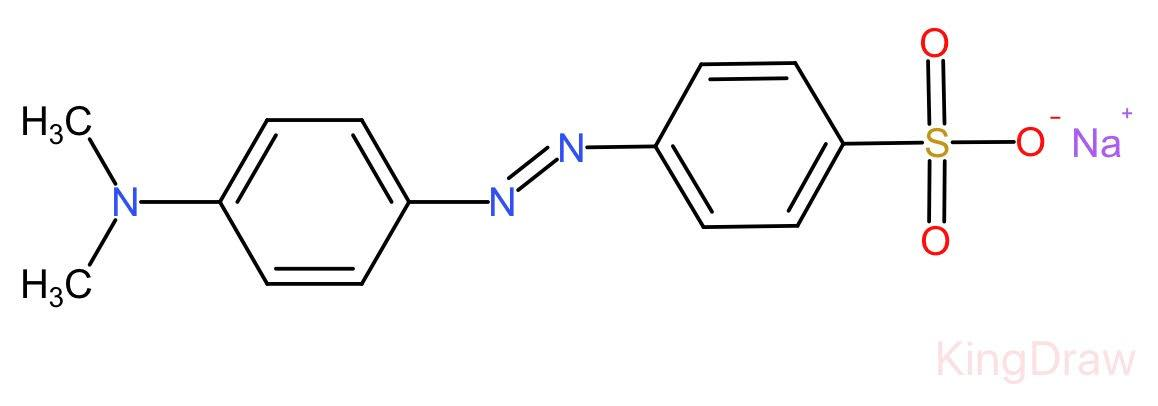
Unlike a universal indicator, it does not show a full spectrum of colour change but does have a sharp endpoint.
- In a solution that is becoming less acidic in nature, methyl orange tends to change its colour from red to orange and finally to yellow. This entire colour change occurs in acidic conditions.
- Methyl orange shows a red colour when the pH of the solution is less than or equal to 3.1 and shows a yellow colour when the pH is above or equal to 4.4.
- As the changes occur in the $p{k}_{a}$ of a mid-strength acid, methyl orange is usually used for the titration of acids.
Therefore, in the acidic medium, it is red in colour and in the basic medium, it is yellow in colour.
Hence, option (b) is the correct answer.
Note: Methyl orange is insoluble in diethyl ether, but its solubility in water is 0.5 g/100mL (20 degree Celsius). It is an orange solid in appearance and direct contact with methyl orange should be avoided.
Complete step by step answer: Methyl orange is a chemical compound having chemical formula ${C}_{14}{H}_{14}{N}_{3}Na{O}_{3}S$.

Unlike a universal indicator, it does not show a full spectrum of colour change but does have a sharp endpoint.
- In a solution that is becoming less acidic in nature, methyl orange tends to change its colour from red to orange and finally to yellow. This entire colour change occurs in acidic conditions.
- Methyl orange shows a red colour when the pH of the solution is less than or equal to 3.1 and shows a yellow colour when the pH is above or equal to 4.4.
- As the changes occur in the $p{k}_{a}$ of a mid-strength acid, methyl orange is usually used for the titration of acids.
Therefore, in the acidic medium, it is red in colour and in the basic medium, it is yellow in colour.
Hence, option (b) is the correct answer.
Note: Methyl orange is insoluble in diethyl ether, but its solubility in water is 0.5 g/100mL (20 degree Celsius). It is an orange solid in appearance and direct contact with methyl orange should be avoided.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




