
Complete the following equation.
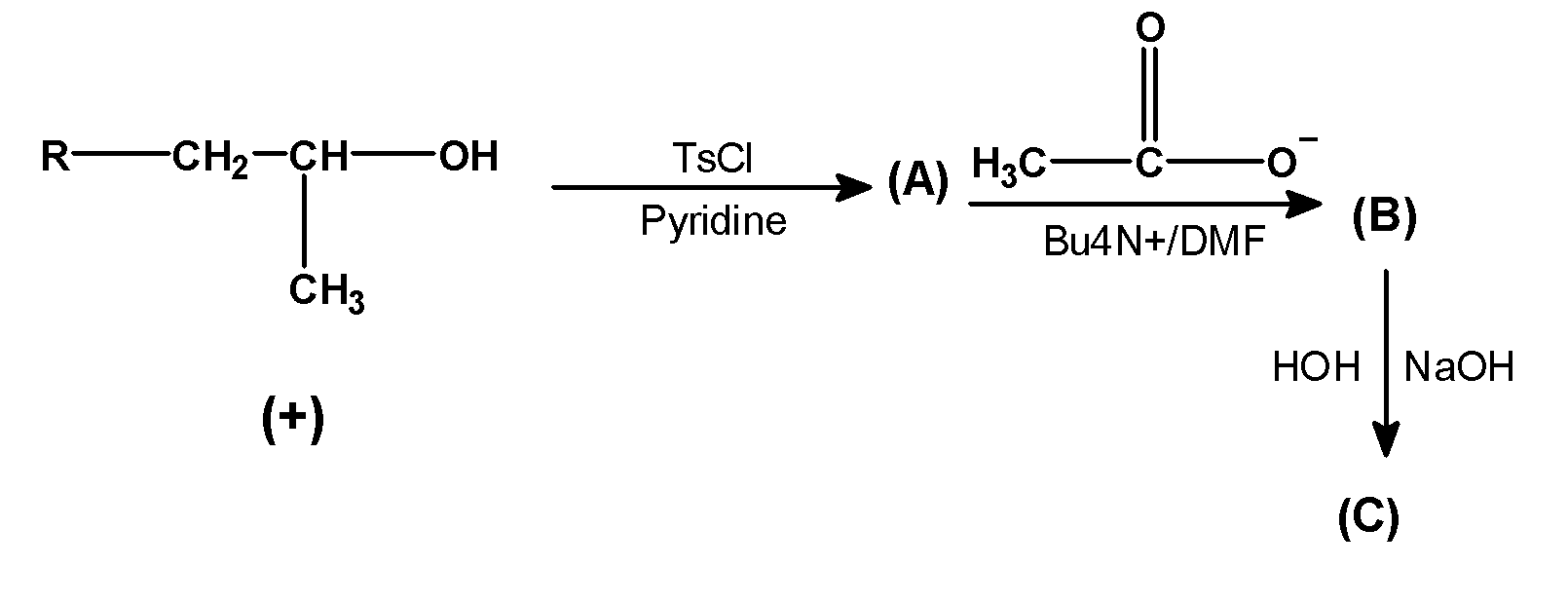

Answer
573.6k+ views
Hint: TsCl is an organic reagent. It converts the hydroxyl group into the better leaving group. The reaction of an alcohol with TsCl converts the hydroxyl group into the tosyl group. Tosyl is a good leaving group. Thus the attack of acetate ion pushes out the tosyl group.
Complete Solution :
TsCl or p-toluenesulfonyl chloride is a reagent that converts the hydroxyl group $\text{ (}-\text{OH) }$ into the leaving group. The alcohols are treated with the TsCl in presence of weak bases such as pyridine. This results in the sulfonate esters. The secondary alcohol given in the question reacts with TsCl or p-toluenesulfonyl chloride in presence of weak base pyridine .this reaction converts $\text{ (}-\text{OH) }$ group into $\text{ (}-\text{OTs) }$ the group.
- The product (A) that is the tosyl group is treated with the acetate ion in presence of tetra butyl amine in DMF. This reaction replaces the tosyl group with acetate ion. Tosyl group is a good leaving group. It tends to leave the molecule .thus in the presence of an incoming group acetate ion it is removed from the molecules. The obtained product is (B)
- The product (B) is further treated with the water molecules and alkali i.e sodium hydroxide. This replaces the acetate ion with the hydroxide ion. Since hydroxide is a good nucleophile it pushes out the acetate group. This is the product(C).
- From reactant to product (C) we can say that secondary alcohol remains the same only the difference in the stereochemistry of the molecule. Acetate ion attacks on the carbon atom bearing tosyl group form the backside which pushes out the tosyl group with inversion of configuration. Thus we have shown the groups on the opposite side. The reaction progress is as shown below,
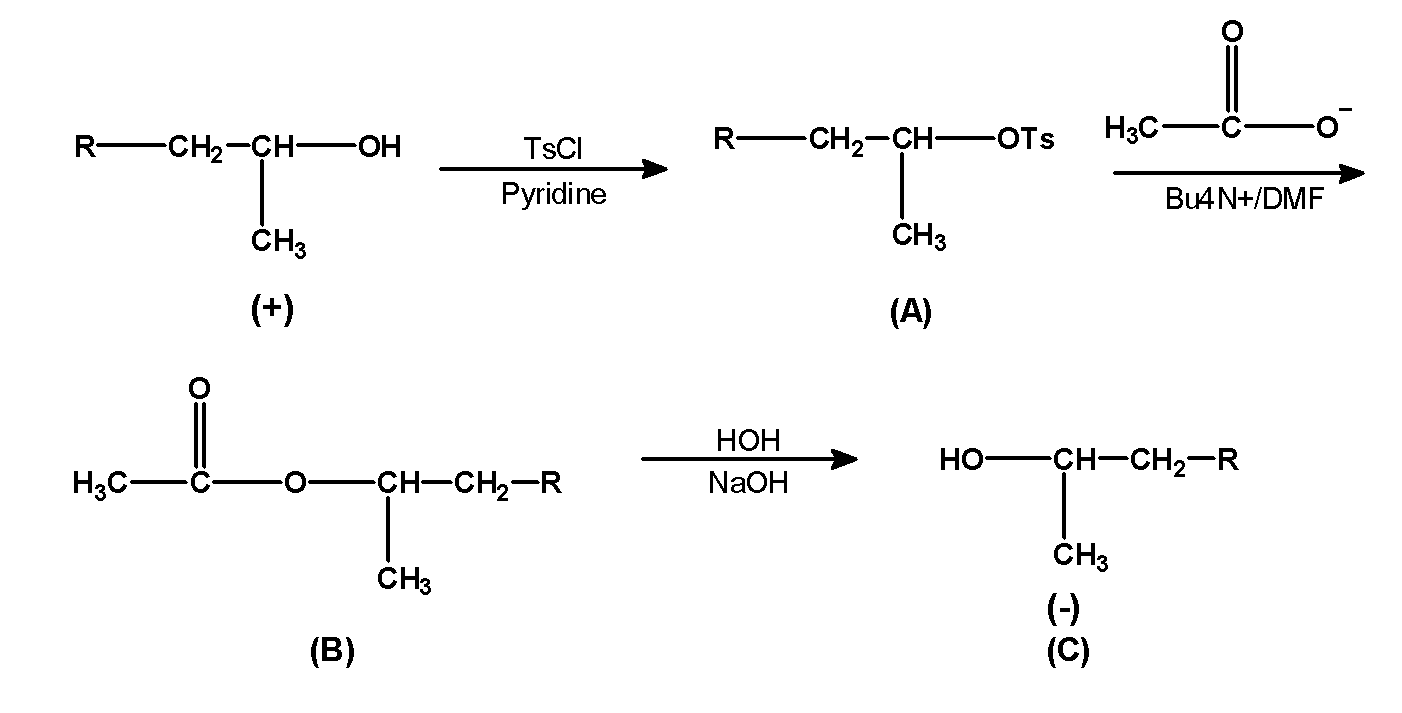
Note: Note that in the action of TsCl the purpose of the pyridine is to remove the hydrochloric acid formed during the reaction and hydroxide groups are strong bases and therefore they are a poor leaving group. But TsCl converts it into a good leaving group. In simpler words the product B is obtained by nucleophilic bimolecular substitution reaction.
Complete Solution :
TsCl or p-toluenesulfonyl chloride is a reagent that converts the hydroxyl group $\text{ (}-\text{OH) }$ into the leaving group. The alcohols are treated with the TsCl in presence of weak bases such as pyridine. This results in the sulfonate esters. The secondary alcohol given in the question reacts with TsCl or p-toluenesulfonyl chloride in presence of weak base pyridine .this reaction converts $\text{ (}-\text{OH) }$ group into $\text{ (}-\text{OTs) }$ the group.
- The product (A) that is the tosyl group is treated with the acetate ion in presence of tetra butyl amine in DMF. This reaction replaces the tosyl group with acetate ion. Tosyl group is a good leaving group. It tends to leave the molecule .thus in the presence of an incoming group acetate ion it is removed from the molecules. The obtained product is (B)
- The product (B) is further treated with the water molecules and alkali i.e sodium hydroxide. This replaces the acetate ion with the hydroxide ion. Since hydroxide is a good nucleophile it pushes out the acetate group. This is the product(C).
- From reactant to product (C) we can say that secondary alcohol remains the same only the difference in the stereochemistry of the molecule. Acetate ion attacks on the carbon atom bearing tosyl group form the backside which pushes out the tosyl group with inversion of configuration. Thus we have shown the groups on the opposite side. The reaction progress is as shown below,

Note: Note that in the action of TsCl the purpose of the pyridine is to remove the hydrochloric acid formed during the reaction and hydroxide groups are strong bases and therefore they are a poor leaving group. But TsCl converts it into a good leaving group. In simpler words the product B is obtained by nucleophilic bimolecular substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




