
Compound \[[A]\] is an aromatic amine which reacts with \[NaN{O_2} + HCl\] at \[273 - 278K\] and forms the compound \[\left[ B \right]\]. Compound \[\left[ B \right]\] react with \[HB{F_4}\] and obtain the product on further heating, in the presence of \[NaN{O_2}\] and confirm compound \[[C]\]. Compound \[[C]\] reduced in the presence of \[Sn/HCl\] to reform the compound \[[A]\]. Write the general name of ' \[A\]’,'\[B\] ' and '\[C\]' and write the equation of all reactions involved?
Answer
595.5k+ views
Hint:You can figure out what the compounds are by looking at all the reactions and figure out a starting material that can undergo all the following reactions. You can start the solution by describing stepwise reactions with properly labelled diagrams. Then follow by summarizing all the reactions in one diagram.
Complete step-by-step answer:
The reactions occur in the following steps –
Step 1 – Aniline reacts with \[NaN{O_2}\] and \[HCl\] at \[273 - 278K\] to form Benzene diazonium chloride. Aniline is a primary aromatic amine. Aniline is a compound which has an amino (\[N{H_2}\]) group attached to a benzene ring. The reaction is shown below
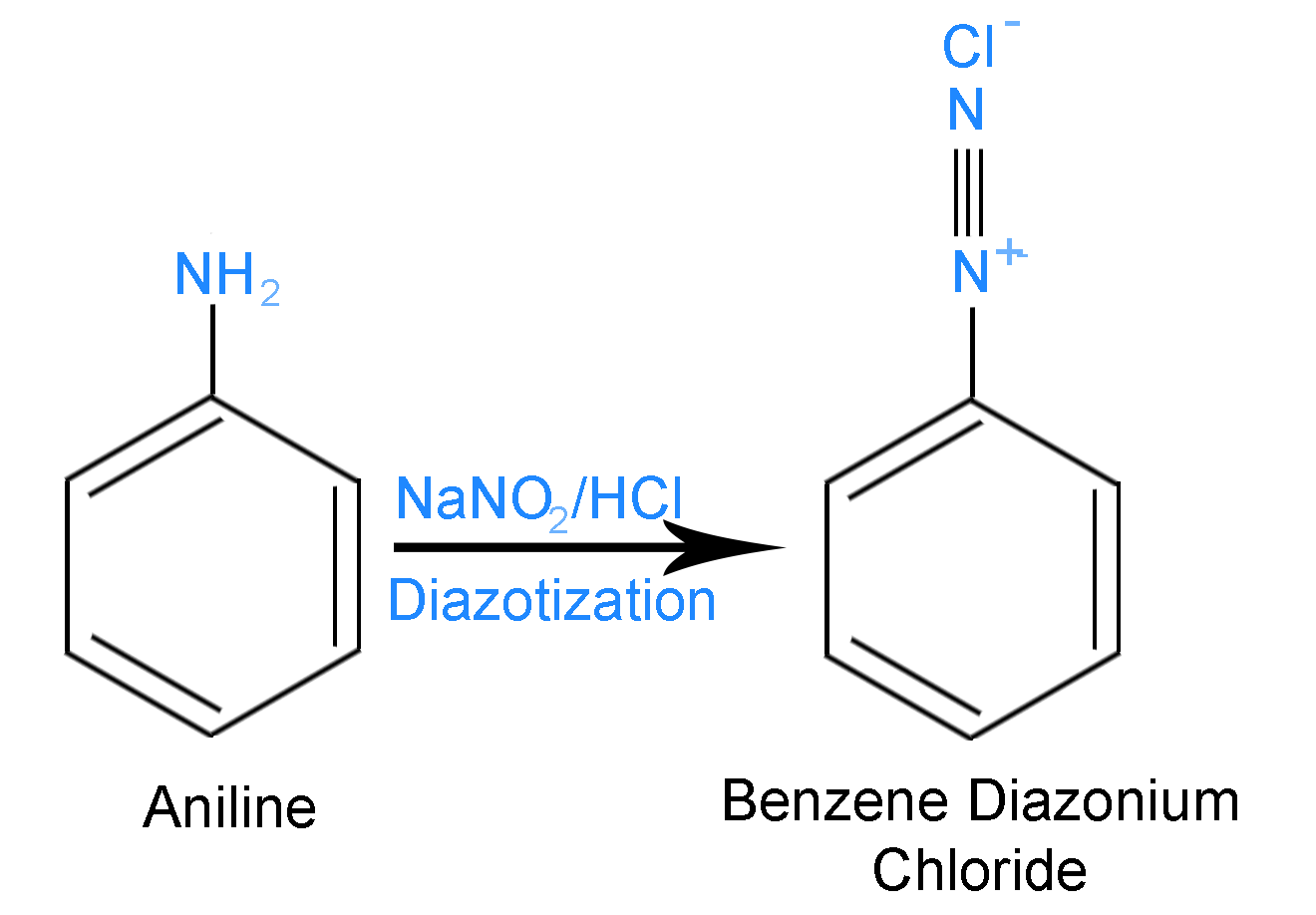
Aniline is the compound \[[A]\] and Benzene Diazonium Chloride is the compound \[\left[ B \right]\].
Step 2 - Benzene Diazonium Chloride reacts with \[HB{F_4}\] in the presence of heat forms fluorobenzene. Fluorobenzene on further heating with \[NaN{O_2}\] forms Nitrobenzene as the product. This reaction is shown below
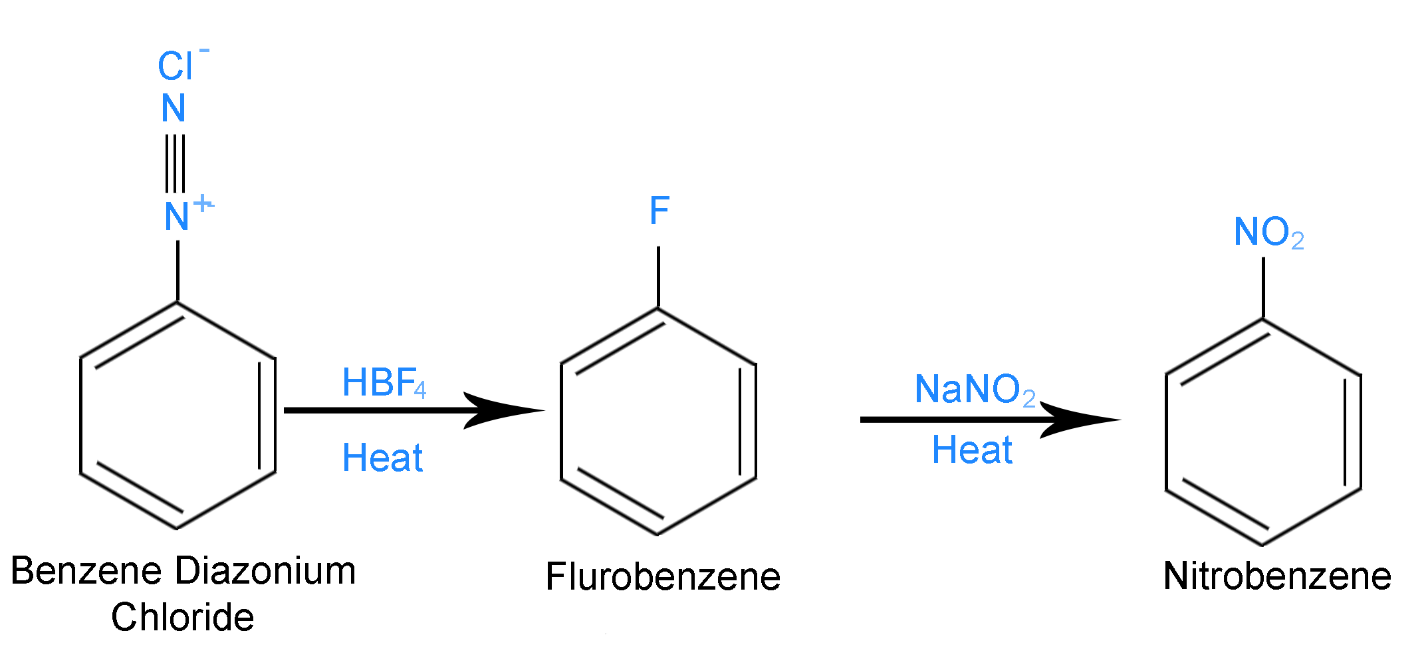
Nitrobenzene is the compound \[[C]\] mentioned in the problem.
Step 3 – Nitrobenzene is reduced in the presence of \[Sn/HCl\] to form Aniline.
This reaction is shown below
The whole reaction is summarized in the following diagram
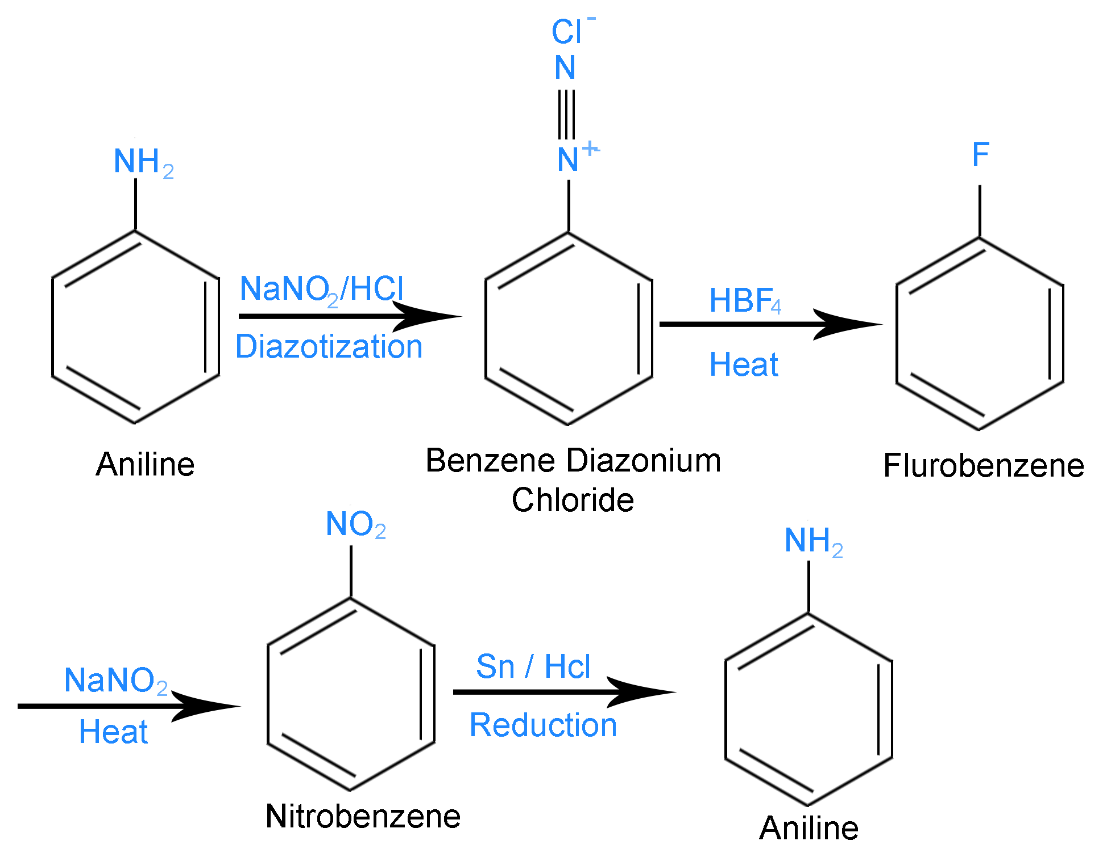
Hence, Aniline is the compound \[[A]\],Benzene Diazonium Chloride is the compound \[\left[ B \right]\] and Nitrobenzene is the compound \[[C]\] mentioned in the problem.
Note: The reaction used in step 1 is used in making dyes and pigments. The reaction in step 2 is used to make fluorobenzene which is very important in controlling the carbon content in steel manufacturing. The second reaction in step 2 is used to produce nitrobenzene which is heavily used in lubricating oils in motors and machinery.
Complete step-by-step answer:
The reactions occur in the following steps –
Step 1 – Aniline reacts with \[NaN{O_2}\] and \[HCl\] at \[273 - 278K\] to form Benzene diazonium chloride. Aniline is a primary aromatic amine. Aniline is a compound which has an amino (\[N{H_2}\]) group attached to a benzene ring. The reaction is shown below

Aniline is the compound \[[A]\] and Benzene Diazonium Chloride is the compound \[\left[ B \right]\].
Step 2 - Benzene Diazonium Chloride reacts with \[HB{F_4}\] in the presence of heat forms fluorobenzene. Fluorobenzene on further heating with \[NaN{O_2}\] forms Nitrobenzene as the product. This reaction is shown below

Nitrobenzene is the compound \[[C]\] mentioned in the problem.
Step 3 – Nitrobenzene is reduced in the presence of \[Sn/HCl\] to form Aniline.
This reaction is shown below

The whole reaction is summarized in the following diagram

Hence, Aniline is the compound \[[A]\],Benzene Diazonium Chloride is the compound \[\left[ B \right]\] and Nitrobenzene is the compound \[[C]\] mentioned in the problem.
Note: The reaction used in step 1 is used in making dyes and pigments. The reaction in step 2 is used to make fluorobenzene which is very important in controlling the carbon content in steel manufacturing. The second reaction in step 2 is used to produce nitrobenzene which is heavily used in lubricating oils in motors and machinery.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




