
How will you convert Benzene into acetophenone?
Answer
562.5k+ views
Hint: In the conversion of benzene into acetophenone electrophilic substitution is taking place where an acyl group is attached to the benzene ring. The name reaction involved in this reaction is the Friedel-Craft reaction.
Complete step by step answer:
Friedel-Craft reaction is a coupling reaction where electrophilic aromatic substitution takes place where an electrophilic group is attached to the aromatic ring.
Friedel-Craft acylation reaction is a type of Friedel-Craft reaction where an acyl group is attached to the aromatic ring. The Friedel-Craft acylation involves addition of acid chloride with the help of Lewis acid catalyst aluminium chloride \[AlC{l_3}\].In Friedel-Craft acylation reaction, ketone is formed as the main product.
The reaction involved in the conversion of Benzene into Benzophenone is Friedel-Craft acylation. In this reaction benzene is treated with acetyl chloride in presence of anhydrous aluminum chloride which acts as a catalyst.
The reaction between Benzene into acetophenone is shown below.
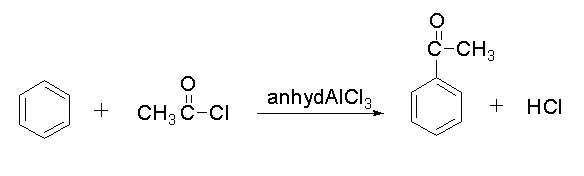
In this reaction, benzene reacts with acetyl chloride in presence of anhydrous chloride to form acetophenone and hydrochloric acid.
In this reaction, first the lewis acid catalyst reacts with acyl chloride to form complex where the acyl chloride loses its chloride ion and form acylium ion \[RC{O^ + }\] which act as an electrophile and stabilized by resonance. The acylium ion then attacks the benzene ring forming an intermediate complex which deprotonate and proton joins with the chloride to form hydrochloric acid.
Note: In Friedel-Craft acylation reaction, acid anhydride can also be used in place of acyl halide. In Friedel-Craft acylation reaction benzene reacts with acid anhydride in presence of anhydrous aluminium chloride to form acetophenone and acetic acid.
Complete step by step answer:
Friedel-Craft reaction is a coupling reaction where electrophilic aromatic substitution takes place where an electrophilic group is attached to the aromatic ring.
Friedel-Craft acylation reaction is a type of Friedel-Craft reaction where an acyl group is attached to the aromatic ring. The Friedel-Craft acylation involves addition of acid chloride with the help of Lewis acid catalyst aluminium chloride \[AlC{l_3}\].In Friedel-Craft acylation reaction, ketone is formed as the main product.
The reaction involved in the conversion of Benzene into Benzophenone is Friedel-Craft acylation. In this reaction benzene is treated with acetyl chloride in presence of anhydrous aluminum chloride which acts as a catalyst.
The reaction between Benzene into acetophenone is shown below.

In this reaction, benzene reacts with acetyl chloride in presence of anhydrous chloride to form acetophenone and hydrochloric acid.
In this reaction, first the lewis acid catalyst reacts with acyl chloride to form complex where the acyl chloride loses its chloride ion and form acylium ion \[RC{O^ + }\] which act as an electrophile and stabilized by resonance. The acylium ion then attacks the benzene ring forming an intermediate complex which deprotonate and proton joins with the chloride to form hydrochloric acid.
Note: In Friedel-Craft acylation reaction, acid anhydride can also be used in place of acyl halide. In Friedel-Craft acylation reaction benzene reacts with acid anhydride in presence of anhydrous aluminium chloride to form acetophenone and acetic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




